Neuroablative Intervention for Refractory Obsessive-Compulsive Disorder
Article information
Abstract
Objective
This review aims to investigate the progression of neuroablation, along with documented clinical efficacy and safety, in the management of treatment-resistant obsessive-compulsive disorder (OCD).
Methods
We searched and compiled clinical research results of neuroablation therapy reported to date. We extracted outcomes related to clinical efficacy, side effects, and surgical complications. Additionally, we summarized key claims and findings.
Results
Neuroablative intervention is a potential treatment approach for refractory OCD. Recent advancements, such as real-time magnetic resonance monitoring and minimally invasive techniques employing ultrasound and laser, offer distinct advantages in terms of safety and comparative efficacy when compared to conventional methods. However, the absence of randomized controlled trials and long-term outcome data underscores the need for cautious consideration when selecting neuroablation.
Conclusion
Neuroablative intervention shows promise for refractory OCD, but vigilant consideration is essential in both patient selection and surgical method choices due to the potential for rare yet serious complications.
INTRODUCTION
Obsessive-compulsive disorder (OCD), a neuropsychiatric disease, can become a lasting illness despite several therapeutic strategies that affect not only patients themselves but also their families [1]. Neurosurgical intervention is a potential treatment option for persistent, treatment-resistant OCD that falls into two main categories: neuroablation and deep brain stimulation (DBS) [2]. Although both approaches aim to modulate the dysfunctional brain circuit associated with OCD, known as the cortico-striato-thalamo-cortical circuit, there are differences in accessibility and side effects, and each approach has its unique values [3,4]. Neuroablation, lesioning, or damage to specific brain areas has a longer history and experience than DBS for severe and treatment-resistant OCD. Recent meta-analyses have suggested that neuroablation has comparative efficacy and added benefits of cost-effectiveness and safety compared with DBS for treatment-resistant OCD [5-7]. Nonetheless, there are notable obstacles faced by both patients and medical practitioners when considering neuroablation, including a lack of comprehensive understanding of its mechanisms, doubtful efficacy, occasional serious adverse effects, and prejudges from history [8,9].
Recently, the expanding knowledge of neural circuitry abnormalities in OCD and advancements in minimally invasive techniques have attracted interest in neuroablation for OCD [10-12]. Converging evidence has been published that restores the dysfunctional global brain network and regional brain activity after neuroablative intervention in patients with intractable OCD [13,14]. Enhanced imaging technologies and an understanding of the pathophysiological circuitry involved in treatment-resistant OCD allow for the precise targeting of problematic pathways, thus maximizing therapeutic results [10,15]. In particular, stereotaxic neurosurgical techniques with realtime surgical monitoring, such as magnetic resonance-guided focused ultrasound (MRgFUS) or MR-guided-laser interstitial thermal therapy (LITT), can minimize the impact on non-target pathways, thus reducing the associated side effects [16,17].
In this review, we chronicle the history of neuroablation procedures for the treatment of OCD and assess their effects and potential complications. Traditionally, four main targets have been used for lesioning procedures in treatment-resistant OCD: the anterior limb of the internal capsule (ALIC; anterior capsulotomy), anterior cingulate cortex and cingulum fibers (cingulotomy), frontothalamic fibers (subcaudate tractotomy), and a combination of anterior cingulotomy and subcaudate tractotomy (limbic leucotomy) [4]. However, anterior capsulotomy was the most frequently performed ablative surgery among the options [18], and the recent MR-guided ablation studies have primarily targeted the internal capsule. In this article, we discuss capsulotomy for simplicity. Cingulotomies and leukotomies were also examined where relevant.
DEVELOPMENT OF NEUROABLATION FOR OCD
Beginning of psychosurgery
Psychosurgery was introduced in the early 1900s before the emergence of psychopharmacology. In 1936, Moniz published his experiences of prefrontal leukotomies for severe depression, anxiety, and aggression, performed by freehand injections of absolute alcohol into the frontal lobes, resulting in substantial symptom improvement in 14 of 20 patients [19]. He reported additional cases, received the Nobel Prize in Medicine in 1949, and coined the term “psychosurgery.” [20] However, at the time, the standards for reporting adverse events were loose, and Moniz did not report even a single adverse event [19], making it difficult to accept the results from a modern medical perspective. Freeman and Watts [21] described their prefrontal lobotomy with the transorbital approach, which is used to surgically interrupt the frontal lobe white matter tracts. Transorbital leukotomy became widely practiced as Freeman traveled across the United States. Before the development of psychopharmacology, prefrontal lobotomies were widely performed across the United States when skepticism about the treatment of mental illness was prevalent. In a review of 10,365 prefrontal lobotomy operations performed between 1943 and 1954, Tooth and Newton confirmed a 70% rate of improvement but also found a mortality rate of 6%, a rate of post procedure new-onset epilepsy of 1%, and post-procedure marked disinhibition in 1.5% of patients. However, the occurrence of unpredictable side effects has criticized the ethics and scientific basis of treatment. Movement against lobotomy was observed. In 1950, some countries, including the Soviet Union, Japan, and Germany, banned lobotomy [22]. In 1967, Freeman was banned from performing further lobotomies after one of his patients experienced a fatal brain hemorrhage.
Development of modern stereotactic neuroablation
In the late 1940s, the advent of frame-based stereotactic procedures enabled the development of more precise and reliable neurosurgical lesions [23]. These procedures have become significantly used since the 1970s, allowing surgeons to accurately target specific brain areas using three-dimensional imaging and computer guidance [24]. The primary goal was to physically alter the brain regions and circuits associated with OCD by creating lesions. Lesion procedures for treatmentresistant OCD mainly target four areas: anterior capsulotomy (ALIC), cingulotomy (anterior cingulate cortex and cingulum fibers), subcaudate tractotomy (frontothalamic fibers), and limbic leukotomy (a combination of anterior cingulotomy and subcaudate tractotomy) [4,25]. The effects and side effects at each surgical site differed slightly; however, overall, they were similar [12], suggesting that these surgical sites are brain structures associated with the same CSCT circuit [26]. Owing to the successful use of DBS in the ALIC in the treatment of OCD, the internal capsule has recently become a popular target.
Capsulotomy for OCD during this period employed two surgical techniques: radiofrequency (RF) and radio-surgical ablation [27]. RF capsulotomy involves a standard stereotactic technique in which RF electrodes are introduced into the ventral ALIC for thermal ablation using fixed parameters [28,29]. Advantages include short operative time and a relatively uncomplicated procedure, in addition to low procedural cost; however, although stereotaxic techniques have been employed, RF ablation still carries unavoidable risks associated with craniotomy, including intracranial hemorrhage (ICH), damage to surrounding brain tissue, or procedural infections. Additionally, it cannot be confirmed whether the lesion has been created successfully during the procedure.
Radiosurgical ablation has been developed as a noninvasive alternative to RF capsulotomy. The first radiosurgical capsulotomy was performed in 1976 for treatment-resistant OCD and other anxiety disorders and has since been conducted widely [30]. In this study, lesions were created by directing radiation from a Cobalt-60 source to specific brain sites, guided by MR and computed tomography images. The main advantage of this procedure is its less invasive nature, as it does not require opening of the skull or causes minimal damage to the surrounding brain tissue, thus reducing immediate postoperative adverse events [24]. A nonrandomized comparison of 25 patients with treatment-refractory OCD at the Karolinska Institute who underwent either RF or radiosurgical capsulotomy between 1988 and 2000 suggested that RF capsulotomy is associated with greater adverse effects than is radiosurgical capsulotomy [28]. However, radiosurgical procedures require a longer time for the lesion to form, making it difficult to confirm the results after the procedure and require a longer time for symptom improvement. There were cases where reoperation was needed due to insufficient lesion formation. Additionally, potentially serious adverse events such as radiationinduced necrosis and cyst formation can be associated with radiation. A detailed summary of the history and clinical experience of radiosurgical capsulotomy has been provided in another review [24].
Updated MR-guided neuroablation
Recently, more precise and less invasive neuroablative surgical techique with MRI guidance have developed. In MRIguided neuroablation, MRI was performed to confirm the creation of lesions at the appropriate location and size. MRguided-LITT is a minimally invasive ablative technique. MRguided-LITT involves the insertion of an optical wire through a burr hole into the target structure and activating laser illumination at the tip to heat and ablate the tissue [31]. Compared to RF thermal lesions, LITT has the advantage of being able to clearly see the target, and obtain a lesion estimate through thermography. Currently, LITT is most widely applied to epilepsy and brain tumors. After the first case report in 2016 [32], two case series involving 18 patients have been reported (Table 1) [33-36].
MRgFUS uses multiple acoustic energy sources within a specially designed helmet to create a lesion under real-time MRI guidance (Figure 1) [37]. By contrast with LITT, in MRgFUS craniotomy is not required. It also allows for ongoing patient condition monitoring and early identification of intra-operative side effects [38]. The results of the two prospective clinical trials have been reported [35,36]. MRgFUS has been widely applied for the treatment of various movement disorders (essential tremors, etc.). Both techniques have shown an efficacy comparable to that of traditional surgical methods but with their minimally invasive nature and real-time monitoring capabilities. In a cost-effectiveness analysis that considered effectiveness, side effects, and follow-up treatment process, MRgFUS was reported to be more advantageous than RF capsulotomy [39].
CLINICAL EFFICACY OF NEUROABLATION
Since most of the research results regarding neuroablation for OCD are retrospective case series with variable outcome criteria and observation periods, the effect could be determined from a systematic review and meta-analysis. A recent systematic review of observational studies on capsulotomy for OCD reported that, after a 12-month follow-up, the mean reduction in the OC symptoms which measured with Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score and full response (≥35% reduction in Y-BOCS) rate were 37% and 41%, respectively [40]. In another meta-analysis of the effectiveness of neuroablation treatment for OCD, the overall response rate for neuroablation treatment was 55% at the last follow-up, with observed response (≥35% reduction in Y-BOCS) rates of 59%, 36%, and 47% for capsulotomy, cingulotomy, and limbic leucotomy, respectively [18]. There was significant heterogeneity between groups for different surgeries; however, the heterogeneity within each surgery group was small.
There is only one double-blind-randomized controlled trial (RCT) that observed the efficacy and safety of capsulotomy using radiosurgical ablation [41]. Out of 16 participants with refractory OCD, eight underwent actual gamma ventral capsulotomy and eight underwent placebo surgery, and blinding was maintained for 12 months. During the 12 months after surgery, the median Y-BOCS score decreased by 28.6% in the active treatment group and 5.8% in the sham group. At 12 months, two patients in the active treatment group (25%) were responders (≥35% reduction in Y-BOCS) but none of in the sham group. Here, the response rate (25%) in blinded portion (12 months) was lower than that in previous open studies and was not statistically significant. However, the response rate increased to 62.5% at 54 months, since 3 additional patients in the active group improved continuously became responder.
Two separate case series, involving a total of 18 patients, have reported on the use of MR-guided LITT capsulotomy. McLaughlin et al. [34] presented their findings on the treatment of refractory OCD using LITT-capsulotomy. Out of the 9 patients who agreed to follow-up, 7 were classified as full responders, with a Y-BOCS reduction of at least 35% at the last follow-up. Interestingly, 2 patients who did not respond to gamma knife capsulotomy previously achieved a positive response with LITT-capsulotomy. Another case series by Satzer et al. [33] included 18 patients with refractory OCD, one of whom did not respond to DBS surgery. These patients underwent MR-guided LITT anterior capsulotomy. At the last follow-up, which ranged from 3 to 51 months, 11 patients (61%) showed a positive response. The patient who had previously received ineffective DBS treatment demonstrated a 77% improvement in Y-BOCS scores. Overall, the responses to LITT-capsulotomy were comparable to those observed with stereotactic radiosurgery, but the time taken for symptom improvement was shorter in the LITT group.
Recently, two open-label trials on MRgFUS capsulotomy were conducted, in which planned measures were used to observe the outcome in a prospective design [35,36]. Kim et al. [35] reported the long-term results of MRgFUS capsulotomy in 11 patients with treatment-resistant OCD. At 24 months, 54.6% of patients (6/11) were responders (≥35% reduction in Y-BOCS), and the mean Y-BOCS score decreased from 34.4 (±2.3) at baseline to 21.8 (±4.8) and 21.3 (±6.2) at 12 and 24 months, respectively [36]. Davidson et al. [36] conducted phase I trials of MRgFUS capsulotomy for treatment-resistant OCD and depression. Although the follow-up period varied from 6 to 12 months, four of six patients were classified as the response group at the last follow-up, as their Y-BOCS decreased by more than 35%.
ADVERSE EFFECTS OF NEUROABLATION
According to a systematic review neuroablation with 476 cases in 26 studies, most of them were mild and transient, the top 3 most frequently reported were postoperative headache (incidence 14.9%), cognitive deficits (9.1%), and behavior problems (8.1%). However, the incidence and characteristics differed depending on the surgical methods. However, rare but permanent adverse effects have hurled the choice.
ICH is a surgical adverse event that primarily occurs in mechanical neuroablation techniques like RF and LITT, which involve the insertion of probes or fibers through craniotomy. The incidence of ICH was up to 20% during the era of mechanical leukotomies, and there were cases resulting in death [42]. However, in recent RF ablation procedures ICH the incidence and severity of ICH have decreased to around 1%, with no lasting consequences. In two recent studies on MR-guided LITT [33,34], one asymptomatic ICH was reported in each study, but it resolved spontaneously within three months without any long-term effects.
Radiosurgical capsulotomy can lead to brain cysts or persistent brain edema as potential adverse events [24]. A review indicates a 5% risk of radiation-induced delayed cyst formation, which can occur up to five years after the surgery [18]. These cysts are often symptomless but may necessitate medical or surgical intervention if they become symptomatic.
Concerns about frontal lobe syndrome or cognitive and personality changes following neuroablation do not appear to be well-substantiated based on literature review [18,42]. Cognitive and frontal lobe function are assessed using various neuropsychological measures. Rück et al. [28] reported significant frontal lobe dysfunction measured through the Execution, Apathy, and Disinhibition Scale and neuropsychological tests after surgery. However, as there was no preoperative evaluation, it is unclear whether this is a side effect of the surgery. Additionally, a RCT study on gamma capsulotomy did not observe any permanent detrimental neuropsychological or personality changes over a period of five years [41]. Apathy has been reported in 22%–40% of patients undergoing anterior capsulotomy, but it is often temporary and associated with multiple procedures and high radiation doses, rather than lesion volume [24]. A recent report on LITT showed that 39% of patients experienced transient postoperative apathy, which resolved on its own [33]. Temporary apathy may be linked to temporary changes in the permeability of the local blood-brain barrier. Interestingly, in the MRgFUS prospective study, frontal lobe dysfuction including apathy was not observed [16].
Weight gain is a well-documented side effect of anterior capsulotomy [18], although it is unclear whether it directly results from the procedure or is a consequence of improved social functioning and increased appetite after treatment. In a study involving DBS of the ALIC and nucleus accumbens for patients with OCD and addiction, variations in weight have also been documented [43,44]. Moreover, DBS is under consideration as a prospective intervention for anorexia [45]. Similarly, in the case series of MRgFUS capsulotomy for treatment-resistant depression [46], notable weight gain were observed over a 12-month period. Therefore, the careful observation of unwanted weight gain is required.
FUTURE DIRECTION FOR PSYCHOSURGERY
Thoughtful consideration for selection criteria
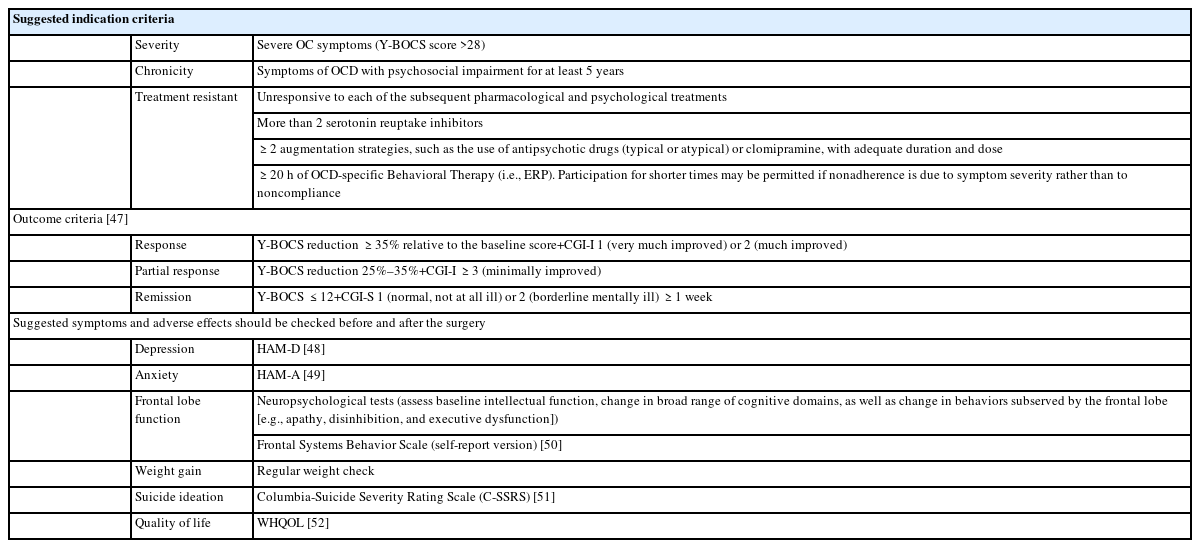
Throughout the past few decades, various studies have employed diverse selection criteria when selecting OCD patients for neurosurgical interventions. In recent capsulotomy clinical trials, symptom severity, chronicity, and treatment resistance have been used as criteria for patient selection (Table 2) [35,41,47-52]. Treatment resistance is typically diagnosed when a patient does not respond to a certain number of psychopharmacological and psychotherapeutic treatments, including electroconvulsive therapy [2]. However, this criterion related to treatment resistance poses a complex challenge, given the constant evolution of medical and non-invasive treatment methods. For example, recent guidelines recommend non-invasive neuromodulation techniques, including deep repetitive transcranial magnetic stimulation (deep rTMS), for the treatment of OCD [2]. Therefore, before considering neurosurgical interventions, it is important to refer or consult with a specialist, such as an OCD expert team or psychiatrist experienced in treating refractory OCD. These specialists can provide a comprehensive evaluation, suggest alternative treatment options, or recommend adjunctive therapies to improve outcomes. Additional general criteria for the presurgical evaluation of psychiatric patients, as well as ethical considerations, were described in a multinational consensus statement [53].
Adherence to treatment is another important issue. It is crucial to assess if patients have adhered to their psychiatrist’s treatment plan correctly and engaged actively in cognitive-behavioral therapy. Certain individuals may sometimes envision that neurosurgical interventions will miraculously alleviate their symptoms, regardless of their personal efforts [54]. Since maintaining medical and behavioral treatment after neurosurgical intervention is required until the optimal surgical effect appears, evaluate adherence to treatment is required not only defining the treatment resistant, but also deciding psychosurgery.
Refining outcome measures
Establishing a scientific and grounded plan to evaluate treatment effectiveness is essential for the development of modern neuroablation. Older literature lacked objective outcome measures, necessitating the use of replicable tools for assessing effectiveness [42]. Recent clinical trials use tools that can evaluate not only the symptoms OCD, such as Y-BOCS [55], but also overall clinical improvement (clinical global improvement) to confirm treatment response (Table 2). Nevertheless, the scales mentioned may be insufficient in capturing the life changes experienced by patients who undergo neurosurgery.
According to a recent qualitative study of capsulotomy experiences [54], factors such as quality of life, personal values, and social functioning are important in the decision-making process and assessment of treatment effects. In particular, for individuals with OCD, even small improvements in symptoms can lead to significant enhancements in their perceived quality of life owing to the substantial subjective distress caused by the symptoms [54,56]. Conversely, there have been reports of significant improvement according to Y-BOCS scores, while the quality of life remained unchanged [57]. Therefore, when assessing treatment effects, it may be necessary to use various scales that reflect mood, impulsivity, suicidality, cognitive function, and so on [9,58]. The same considerations apply to the observation of adverse effects.
The timing of assessing these effects also requires further discussion. In the case of gamma capsulotomy, lesions gradually appear, and improvements in OCD symptoms are reported to occur slowly [24]. However, in studies such as that of Kim et al. [35] involving MRgFUS found that symptom improvement could take up to two years, indicating delayed progress. Moreover, studies observing electrophysiological changes have shown that alterations in electrophysiological connectivity occur gradually over a span of six months [59]. Considering these findings, the evaluation of surgical outcomes should be conducted after a minimum period of at least 12 months following surgery.
Target optimization
The development of an MR-guided procedure and diffusion tensor imaging (DTI) imaging enables a more precise and planned execution of capsulotomy. ALIC is a particularly salient structure for psychiatric surgery because it is a point of convergence for key white matter fibers connecting the prefrontal and anterior cingulate cortices with the hippocampus, amygdala, and thalamus, which together form a core limbic network in the human brain [60]. Therefore, when performing a capsulotomy, the effects and side effects can vary depending on the fiber tract affected by the lesion. Primate fiber-tracking studies and human DTI techniques have revealed how fiber tracts originating from the prefrontal cortex are positioned within the ALIC [61,62]. Additionally, the ventral portion of the ALIC is related to responder status, suggesting that lesions in this region are more likely to produce a clinical response [63]. However, recent research results on treatment location and outcomes are not the same [64], which can be attributed to two factors. First, the fiber tract in the ALIC can exhibit inter-individual variability in neural fiber pathways. Second, each patient may have different abnormalities in neural circuits [65]. Further studies on neurocircuit-based patient selection [10] and use of tractography to guide patient-specific anterior capsulotomy for OCD are needed [66].
CONCLUSION
Advances in biological understanding and precision surgical techniques have broadened the possibilities for use in psychosurgery. Recent neuroablative intervention can be expected to have safer and faster clinical effects. However, to avoid mistakes when psychosurgery is performed indiscriminately without a proper rationale, ongoing research is necessary for appropriate patient selection and evaluation criteria.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Chan-Hyung Kim. Data curation: all authors. Formal analysis: all authors. Methodology: all authors. Project administration: Chan-Hyung Kim. Resources: Chan-Hyung Kim. Software: Jhin Goo Chang. Supervision: Chan-Hyung Kim. Validation: Jhin Goo Chang. Visualization: Jhin Goo Chang. Writing—original draft: Jhin Goo Chang. Writing—review & editing: all authors.
Funding Statement
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI22C0520). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (2021M3E5D9025022). The funding source did not give any influences on the study design, data collection, analysis and interpretation of data, the writing of the report, and the decision to submit the paper for publication.