Effect of Personalized Blue-Enriched White Light Intervention on Rest–Activity and Light Exposure Rhythms in Mild and Moderate Alzheimer’s Disease
Article information
Abstract
Objective
We aimed to examine the effectiveness of personalized light intervention using a blue-enriched light-emitting-diodes device on rest–activity rhythm (RAR) and light exposure rhythm (LER) in patients with mild and moderate Alzheimer’s disease (AD).
Methods
AD patients with poor sleep quality and/or insomnia symptoms were assigned into either an experimental group (EG) or control group (CG) in a single-blind design. Personalized light intervention was given at 9–10 h after individual dim light melatonin onset, lasting for 1 h every day for two weeks in the EG (77.36±5.79 years, n=14) and CG (78.10±7.98 years, n=10). Each patient of CG wore blue-attenuating sunglasses during the intervention. Actigraphy recording at home for 5 days was done at baseline (T0), immediate postintervention (T1), and at four weeks after intervention (T2). The variables of RAR and LER were derived using nonparametric analysis.
Results
We found a significant time effect on the intradaily variability (IV) of RAR at T2 with respect to T0 (p=0.039), indicating reduced IV of RAR at four weeks after personalized light intervention regardless of blue-enriched light intervention. There was a time effect on the IV of LER at T1 with respect to T0 (p=0.052), indicating a reduced tendency in the IV of LER immediately after intervention.
Conclusion
Our personalized light intervention, regardless of blue-enriched light source, could be useful in alleviating fragmentation of RAR and LER in AD patients.
INTRODUCTION
Sleep and behavior disturbances across the day in Alzheimer’s disease (AD) could be described with decreased activity during the day, confused behavior in the evening, and fragmented sleep during the night [1]. These might be related to a diminished sleep-wake rhythmicity, reflecting dysfunction in endogenous circadian system recognized as consequences of degenerative changes in the suprachiasmatic nucleus (SCN) [2], known as the central circadian pacemaker.
There are considerable evidences of circadian abnormalities in AD, including disrupted sleep-wake cycle and impaired rhythms of hormones and body temperature [3]. In particular, disturbances in rest–activity rhythm (RAR) have been well documented in AD patients. They could be manifested with increased daytime naps, increased nocturnal awakenings, and day-night sleep pattern reversals, which are major causes for institutionalization in AD patients [3,4]. Disturbances of the RAR in AD patients are characterized by its reduced stability, increased variability, and reduced amplitude, which are progressively deteriorated corresponding to the disease severity [4,5]. Furthermore, they could be associated with depression and cognitive decline in AD patients [6]. Meanwhile, RAR disturbance as a risk factor for AD may contribute to progression of AD [7]. There are evidences implicating the relationship between the RAR disturbances and AD-related pathologies including hippocampal atrophy [8] and accumulated amyloid β [9]. In a knock-out mouse model, clock disruption has been demonstrated to mediate neuropathological changes in several brain regions [10].
AD patients, particularly institutionalized patients, are known to have little chance of outdoor activity [11]. Thus, they might have insufficient light exposure (i.e., intensity or amount), which is considered an environmental factor that causes their sleep fragmentation. Indeed, there were evidences showing that insufficient environmental light exposure is related to decreased stability of RAR [12], and decreased secretion of nocturnal melatonin [13] known as a circadian regulator of sleep [14] in AD patients. Considering these evidences, light exposure rhythm (LER) in AD patients might be a critical factor in improving their RAR.
Meanwhile, behavioral and psychological symptoms of dementia, such as sundowning, might be a phenomenon that reflects a breakdown of circadian rhythmicity [15,16]. A therapeutic effect of light intervention on sleep and behavior in AD patients may be related to the ability of such intervention to alleviate their circadian disturbances [17]. Moreover, light interventions may have advantages over pharmacological treatments in terms of their side effect profiles for people with dementia [18]. Although some studies have reported the efficacy of light intervention on the RAR of AD patients, findings are inconsistent [5,19,20]. Such inconsistency might be due to the heterogeneity of study subjects (type and severity of dementia) and different modalities of light intervention (wavelength, intensity, duration, and timing) [5,19]. Given that phase-resetting effects of light are dependent on the circadian phase specific to an individual [21], it is essential to establish the appropriate timing of light intervention for each individual. However, most previous studies did not administer light at the appropriate time concerning the timing issue.
In addition, AD patients have revealed phase delays in their rhythms of core-body temperature and activity in contrast to the usual advance of phase observed in older adults [22,23]. Changes in their responsiveness to light in the circadian timing system and neurodegeneration of SCN in AD patients have been postulated as reasons for this. Thus, diminished responses to light can be expected [13], particularly in severely demented patients, whose SCNs are considered to be more degenerated. Considering the above two factors that might hinder the effectiveness of light intervention, our group has already investigated the effectiveness of personalized light intervention based on individual circadian phase using dim light melatonin onset (DLMO), on sleep, cognition, mood, and behavior in patients with mild and moderate AD utilizing the same cohort of AD patients in the present study [24]. In order to reveal the effectiveness of light intervention on the RAR in AD patients, variables were derived with a nonparametric method that could measure the degree of circadian disruption from the frequent nonsinusoidal waveform of rest–activity data. Such variables are known to be more sensitive than cosinor or other parametric variables [25]. In particular, detriments of intradaily variability (IV) indicating the degree of a rhythm fragmentation among nonparametric variables have been often reported in early stages of AD [26], even in preclinical AD [9].
Furthermore, it has been proposed that improvement of cognitive function observed in AD patients after light intervention might be caused by improvement of circadian rhythm [27-30]. However, no study has elucidated the relationship between changes of circadian rhythm and cognitive function following light intervention. Besides, the human circadian system is known to be more sensitive to short wavelength light [31,32]. However, whether short wavelength light can alleviate RAR and LER disturbances in AD patients remains unclear.
Therefore, the aim of this study was to investigate the effectiveness of light intervention based on individual circadian phase using the DLMO on RAR and LER with a portable light box having short wavelength light in patients with mild and moderate AD. Relationships of changes in RAR and LER with changes of cognitive function following light intervention were also explored. Our hypothesis was that our personalized light intervention could have beneficial effects on the RAR and LER and that changes of RAR and LER after the light intervention would be related to changes of cognitive function.
METHODS
The study protocol was approved by the Institutional Review Board at Kangwon National University Hospital, and followed all relevant ethical guidelines and regulations (Approval No. KNUH-2016-10-007-001). It was registered with the Clinical Research Information Service in the Republic of Korea (KCT0005282, registered on 08/04/2020). Written informed consent was obtained from each participant and their legal representatives. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Participants
Participants were referred from the Dementia Clinic at Kangwon National University Hospital between 2018 and 2020. AD were diagnosed by a psychiatrist or a neurologist according to the Diagnostic and Statistical Manual of Mental Disorder, Fifth Edition (DSM-5) [33]. Its severity was based on the Clinical Dementia Rating Scale (CDR) [34]. The eligibility criteria for patients with mild or moderate AD included a diagnosis of probable or possible major neurocognitive disorder AD and a CDR score between 0.5 and 2. Patients who have a Pittsburgh Sleep Quality Index score higher than 5 [35], or the presence of symptoms such as difficulty initiating sleep, maintaining sleep, or early morning awakening for at least three days per week were selected. Patients were excluded from the study if they had: 1) current substance related disorders, depressive disorders, or other psychiatric disorders by DSM-5; 2) current medical illness including liver cirrhosis, chronic pulmonary disease, cancer, uncontrolled diabetes, and uncontrolled hypertension; 3) being suspected or diagnosed with primary sleep disorders except insomnia disorder (i.e., restless legs syndrome, sleep-disordered breathing disorder, hypersomnia, or narcolepsy); 4) a prior history of cerebrovascular disease or central nervous system (CNS) disease or evidence of CNS injury; 5) current use of any medication affecting their circadian rhythms (i.e., Circadin); 6) changes in type or dose of taking hypnotics or CNS active drugs for the study period; 7) significant impairment of hearing ability, visual acuity, or language ability which hindered the completion of neurocognitive tests; and 8) abnormalities in complete blood cell count, liver function test, urine analysis, electrocardiogram, or chest X-ray.
Enrolled patients were further evaluated with self-report questionnaires including the Korean version of Epworth Sleepiness Scale (KESS) [36], the Korean version of the Geriatric Depression Scale [37], and the Korean version of Blessed Dementia Scale-Activity of Daily Living [38]. A clinical interview was conducted for patients with a KESS score 12 or higher to rule out any primary sleep disorder except for insomnia disorder. Prior to light intervention, they underwent a neuro-ophthalmologic examination including visual evoked potentials to assess whether they had pupillary abnormalities or dysfunction of visual pathways. With a single-blind, enrolled patients were assigned into either an experimental group (EG) or a control group (CG) by turns, based on a constant allocation ratio of 1:1 if possible. Fourteen patients (77.36±5.79 years; male [M]:female [F]=2:12) in the EG and 10 patients (78.10±7.98 years; M:F=5:5) in the CG completed our study protocol.
Procedures
Our study protocol is shown in Figure 1. First, enrolled patients were made to wear a wrist actiwatch (Actiwatch 2; Philips Respironics, Murrysville, PA, USA) for five days and the mean sleep onset was determined from actigraphy recording (T0). Second, we began to obtain seven hourly saliva samples in our laboratory under a dim light condition, starting from six hours prior to the sleep onset. DLMO was then determined from salivary melatonin profiles. Third, at home-based setting, using a device (Litebook EDGE; Litebook Company Ltd., Alberta, Canada) emitting mostly short-wavelength radiation, personalized light intervention for one hour per day was applied based on individualized DLMO for two weeks. Actigraphy monitoring for five days was done two more times, immediately post-treatment (T1) and at four weeks after the end of the light intervention (T2). As outcome measures of cognitive function, the Mini-Mental State Examination in the Korean Version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (MMSE-KC) was assessed at T0, T1, and T2. Nonparametric variables of RAR and LER derived from actigraphy data were used as outcomes of light intervention.

Schematic representation of the study protocol. It consists of an intervention (personalized light intervention for two weeks) and three times assessments for outcome variables. T0, baseline; T1, immediately after light intervention; T2, four weeks after light intervention; EG, experimental group; CG, control group; AD, Alzheimer’s disease. PSQI, Pittsburgh Sleep Quality Index; CSDD-K, Korean version of the Cornell Scale for Depression in Dementia; KNPI-Q, Korean version of Neuropsychiatric Inventory Questionnaire; MMSE-KC, Mini-Mental State Examination in the Korean Version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet; TMT-A, Trail Making Test-A; DST, Digit Span Test.
Actigraphy monitoring
Using the Actiwatch 2 with an integrated light sensor, actigraphy recordings along with sleep diary were conducted three times (T0, T1, and T2). We followed the instructions and guidelines of the previous work on this study project for wearing the actiwatch and the quality assessment of the collected data [24].
Saliva melatonin assay
Participants were not allowed to take chocolate, bananas, aspirin, or analgesics on the day of saliva melatonin assay. They were asked to visit our research laboratory by one hour before the starting time of the saliva melatonin assay. During the saliva melatonin assay, they were made to remain in a place with a dim light (less than 15 lux). If necessary, they were allowed to stay with their caregiver during the saliva melatonin assay. We followed the procedures outlined in our previous work for measuring salivary melatonin concentration and determining the DLMO [39]. There was one case as a low secretor. One patient was a low secretor with melatonin values ranging from 1.6 to 2.3 pg/mL. Thus, we could not determine the DLMO. We assumed the DLMO to be half an hour after the last sample time.
Personalized light intervention
Based on the previous study reporting that advance portions of blue light phase response curve have been presented since when nine hours elapsed after DLMO, we determined the administered timing of personalized light intervention between 9 and 10 h after DLMO [40]. We followed the procedures outlined in our previous work on this study project for the application of personalized light intervention [24]. The Litebook EDGE (136 mm×73 mm×16 mm) was used as our light intervention device, which has 60 LED lights emitting light with peaks at 464 nm and 564 nm. Of the energy emitted, 48% is between 420–508 nm and 37% is between 512–616 nm (Figure 2A). Participants were instructed to sit 60 cm away from the device during the intervention and were allowed to engage in other activities, such as reading or listening to music. On the other hand, our controls were made to wear blue-attenuating glasses (Lightguard Hazelnut; Cocoons Live Eyewear Company, San Luis Obispo, CA, USA) during intervention. According to information provided by the manufacturer, the transmittance of blue wavelengths (410 up to 510 nm) is expected to be reduced to less than 10% after wearing blue-attenuating glasses. Transmittances of all other wavelengths are also attenuated (range: 10% to 30%) after wearing these glasses (Figure 2B). Based on measurements, illuminance levels from the device at a distance of 60 cm using a light meter (Testo 545; Testo SE & Co., West Chester, PA, USA) were approximately 30 lux for eyes without these glasses, and approximately 10 lux with these glasses.

The spectral energy distributions of light emitted by the blue-enriched light treatment device (A) and the percentage of light transmitted through the blue-blocked glasses (B).
The compliance of patients to light intervention was monitored by caregivers and the research coordinator through phone interviews. Adverse reactions at the beginning of the light intervention were monitored continuously through phone interviews.
Nonparametric analysis
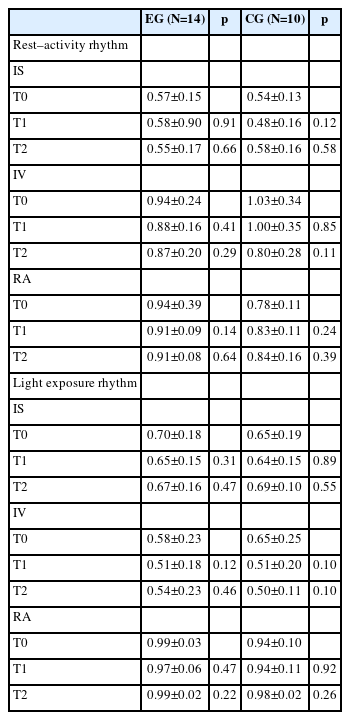
Activity and light exposure data from actigraphy measured at T0, T1, and T2 were analyzed. Each epoch value for activity and light exposure data were computed into hourly bins. Data with hourly bins across 5 days were then used for nonparametric analysis. If bin data were missed among 24-hourly bins, they were interpolated using the mean of values before and after the missing bin. Light exposure data were log-transformed based on their highly skewed empirical distribution, while raw data for the rest–activity have been used [41]. Using R statistical software (package “narACT”) [42], nonparametric analyses of RAR and LER were performed. From these analyses, three nonparametric variables were calculated: interdaily stability (IS), IV, and relative amplitude (RA). IS quantifies the invariability of the rhythm between different days. IV quantifies the frequency and extent of transitions between rest and activity. Higher values indicate increased fragmentation, which may reflect the occurrence of daytime naps and/or nocturnal awakenings [43]. RA was calculated using data from the most active 10-h period (M10) and the least active 5-h period (L5) using the following formula: RA=(M10-L5)/(M10+L5). Higher values indicate a more robust 24 h rhythm, reflecting higher activity during wake and relatively lower activity during the night [43].
Statistical analysis
Baseline characteristics including demographic data were compared between EG and CG using chi-square test for categorical variables and independent t-test for continuous variables as appropriate. IS, IV, and RA of each activity and light exposure data measured at three time points were compared using paired t-tests (T0 vs. T1; T0 vs. T2) in EG and CG, respectively.
Generalized estimating equations (GEE) analyses were performed to examine associations between personalized blue-enriched light intervention and changes of IS, IV, and RA across three time points (T0, T1, and T2). Normal distribution and identity link function were used to estimate 95% confidence intervals. The model was fitted assuming an exchangeable correlation structure with robust standard errors. Main effects of group and time and the interaction of Group×Time were analyzed with GEE. If there were any nonparametric variables with a significant change after light intervention, partial correlation analyses for controlling group were performed to determine relationships between changes in those nonparametric variables and changes in the MMSE-KC score [38].
All statistical analyses were performed using SPSS software package, version 18.0 (SPSS Inc., Chicago, IL, USA). Two-sided p-values of less than 0.05 were considered statistically significant.
RESULTS
Baseline characteristics
Baseline characteristics were similar between EG and CG with respect to age, gender, and education. There were no significant differences in scores of Korean version of Blessed Dementia Scale-Activity of Daily Living [38], Korean version of the Geriatric Depression Scale [37], or MMSE-KC, or distribution of CDR scores between the two groups (Table 1).
Changes in RAR and LER after intervention
In each group, changes in mean±standard deviation of IS, IV, and RA for activity and LERs across T0, T1, and T2 are presented in Table 2. In the EG, there were no significant changes in IS, IV, or RA for activity or LERs from T0 to T1 or from T0 to T2. Likewise, there were no significant changes of those variables in the CG from T0 to T1 or from T0 to T2.
GEE models for changes in RAR and LER
There was no significant time effect or group-by-time interaction on IS or RA among activity rhythm variables. However, we found a significant time effect on IV at T2 with respect to T0 (p=0.039), although group-by-time interaction on IV at T2 was insignificant (p=0.385). Its odds ratio (OR) was 0.814, meaning that IV was reduced by 18.6% in four weeks at the end of intervention compared to that before intervention regardless of the presence of blue-enriched light source.
Similarly, there was no significant time effect or group-by-time interaction on IS or RA among LER variables (p>0.05). However, there was a time effect on IV at T1 with respect to T0, although it did not reach statistical significance (p=0.052). Effect of group-by-time interaction on IV at T1 was not significant either, with an OR of 0.872, meaning that IV was reduced by 12.8% immediately post-intervention compared to that before intervention regardless of the presence of blueenriched light source.
Relationship of the changes in RAR and LER with those in MMSE scores
In total AD patients, there were no significant correlations between changes in IV of RAR or LER after personalized light intervention and changes in the MMSE-KC scores (p>0.05) (Figure 3).
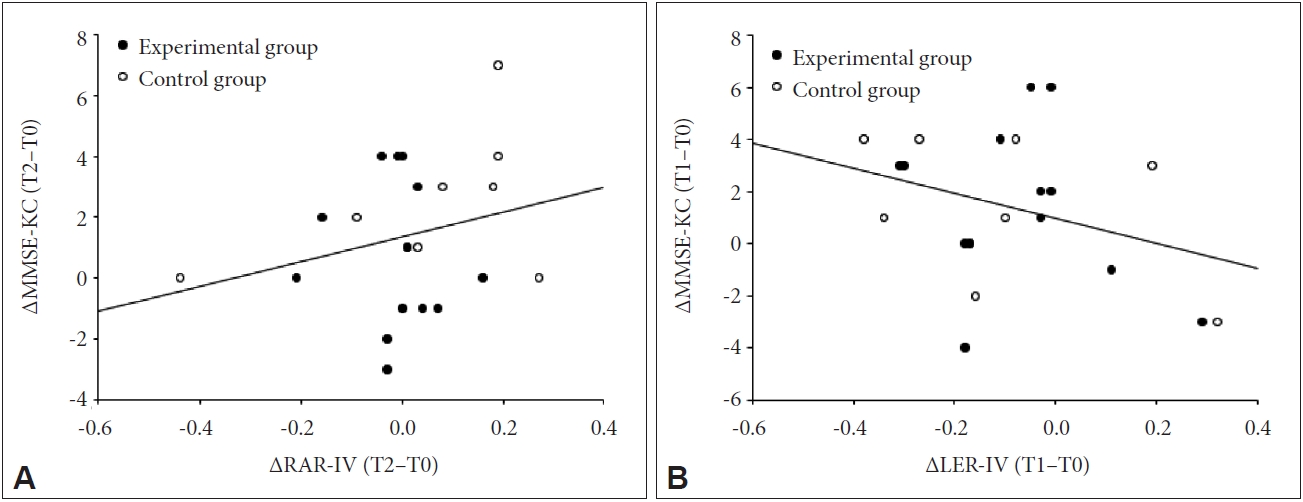
Partial correlation analyses controlling for group in total patients with Alzheimer’s disease (N=24). A: No significant relationship between the differences in the IV of RAR and those in MMSE-KC scores from T0 to T2 (p=0.377, r=0.203). B: No significant relationship between the differences in the IV of LER and those in MMSE-KC scores from T0 to T1 (p=0.149, r=-0.326). IV, intradaily variability; RAR, rest–activity rhythm; MMSE-KC, Mini-Mental State Examination in the Korean Version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet; T0, baseline; T1, immediately after light intervention; T2, four weeks after light intervention; LER, light exposure rhythm.
DISCUSSION
Effect of personalized light intervention on RAR
There were no significant changes in nonparametric variables of the RAR after personalized blue-enriched light intervention (Table 2). However, we found a significant reduction in the IV at the 4-week follow-up after intervention compared to that at baseline (time effect, OR: 0.81, p=0.039) when GEE models were applied to those variables (Table 3). This finding indicates that a personalized light intervention, regardless of blue-enriched light source, can induce a decreased fragmentation of RAR in patients with mild and moderate AD.
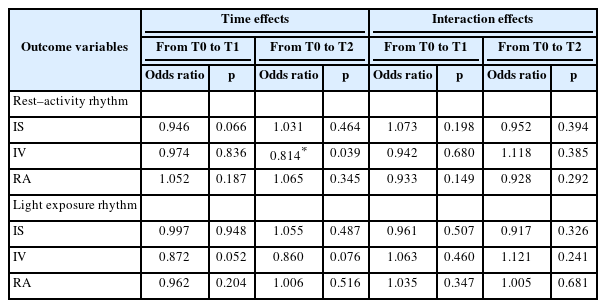
Generalized estimating equations models for changes in the variables of the rest–activity and light exposure rhythms after the timed light intervention (N=24)
Regarding the effectiveness of light intervention on RAR, previous studies have shown conflicting findings possibly due to heterogeneity of study patients with various types of dementia. Contrary to our finding, in a group of patients who were not identified by specific types of dementia, Sloane et al. [44], found no significant changes in IS or IV of RAR after intervention using a blue-white light box in a home-based setting similar to ours. Meanwhile, results of previous studies on a group of AD patients have indicated that the effect of light intervention on the RAR may vary depending on AD severity [27,45,46]. Dowling et al. [45], have applied morning bright light in a group of severe AD patients and found no beneficial effect on their RAR. However, Satlin et al. [46], have reported that evening bright light can ameliorate IV of the RAR in a group of moderate AD patients who might have lower severity of dementia than those in the study of Dowling et al. [45] Together with our finding, these findings implicate that the effectiveness of light intervention on the RAR is likely to be seen in AD patients with lower severity. On the other hand, even in severely demented patients, the effectiveness of light intervention on RAR can vary depending on light intensity or duration [47,48]. Skjerve et al. [48], found an advance of the activity rhythm acrophase in patients with severe dementia throughout high intensity moring light for four weeks, but Fontana Gasio et al. [47], found no significant changes in circadian stability or amplitude characteristics of the RAR, given low intensity dawn–dusk simulation. Furthermore, Yamadera et al. [27], have reported that the number of naps is decreased in patients having a relatively mild AD with a CDR score of 0.5 and 1 after bright light intervention, but not in patients with a CDR score of 2 or 3. Thus, it could be postulated that decreased fragmentation of RAR after light intervention can be expected, especially in patients with less severe AD. Given our patients had mild or moderate AD, our finding might also be in line with this reasoning. Besides, as IV indicates how fragmented a rhythm is throughout the day, the finding that personalized light intervention could alleviate the IV of RAR in our study might be represented by the decreased number of daytime naps and nighttime awakenings in a real situation. There was a recent study that examined phase shifts of DLMO after circadian phase tailored light therapy that was designated with morning light for AD patients with late DLMO, and evening light for those with early DLMO [49]. However, our finding was meaningful as our study administered a light intervention depending on individualized circadian timing and elucidated its potential efficacy on the RAR in AD patients for the first time.
However, we did not identify the better effectiveness of blue-enriched light itself on the RAR compared to that of the control (blue-attenuating light). Thus, our finding should be explained by only the effect of personalized light intervention repeatedly given at a certain time of day, irrespective of blue-enriched light effect. One might argue that we did not find the better effectiveness of blue-enriched light intervention on the RAR compared to that of the control in our study. Contributing factors for this might be as follows. First, this might be attributed to the decreased sensitivity of the circadian system to short wave length light in our patients because of its reduced transmission [31] and/or degeneration of melanopsin ganglion cells with aging [50] that would be expected to be more detrimental in AD patients compared to that in nondemented elderly [51,52]. Second, our patients were unlikely to be certainly disrupted in RARs when comparing values of nonparametric RAR in our patients (the averaged IS=0.55; IV=0.99; and RA=0.86) with those of institutionalized patients reported by previous studies [12,45]. Thus, in our AD patients, it would be difficult to expect further benefits from our blue-enriched light intervention compared to the control due to a ceiling effect. Third, several studies implicated that high light levels (more than 400 lux at the eye of a bluish-white light) for at least 2 h in the morning might be required to increase circadian stimulation [53-56]. However, illuminance levels of our light device delivered at the eyes of the EG patients were only 30 lux measured using a light meter as aforementioned in the method section. As the importance of personalized treatment interventions grows up in AD patients [57], in future studies, it will be necessary to design a light intervention, in which high-intensity light can be delivered at the eyes for AD depending on individualized circadian timing.
In summary, the effect of light intervention on reducing the fragmentation of RAR can be expected, especially in patients with less severe AD, being supported by our finding as well.
Effect of personalized light intervention on LER
There were no significant changes in nonparametric LER variables after the personalized blue-enriched light intervention. However, we found a tendency for decreased IV immediately after intervention compared to that at baseline (time effect, OR: 0.87, p=0.052) when GEE models were applied to those LER variables (Table 3). This finding may indicate that a personalized light intervention, regardless of blue-enriched light source, can immediately decrease the fragmentation of LER in patients with mild or moderate AD, similar to its effect on the RAR.
Undoubtedly, light exposure patterns will appear in relation to activity patterns in a 24-hour environment [12,58,59], particularly in patients who are not restricted in outdoor activity as in our study group. Thus, it was not surprising that our AD patients exhibited alleviation in the IV of both LER and RAR after our personalized light intervention. In the same context as the IV of RAR, increased IV of LER may reflect both diminished light exposure during the day and increased light exposure at night in a real situation. Insufficient light exposure during daytime (low intensity of illumination/decreased duration of bright illumination) has been often reported in AD patients [13,60]. It might be responsible, to some extent, for their poor sleep quality [60] and circadian rhythm disturbance [61,62]. Likewise, increased light exposure at night, which AD patients might experience during awakenings, is expected to have detrimental effects on sleep quality and melatonin secretion [63,64]. Given this, it is noteworthy that our personalized light intervention had a possibility to improve abnormalities in daily patterns of light exposure in AD patients.
Taken together, our findings suggest that personalized light intervention could be effective in reducing fragmented RAR and LER in AD patients. Fragmentation of activity rhythm is noted as one of the most marked circadian abnormalities in AD [65]. It might be due to the underlying misalignment between the activity rhythm and the endogenous circadian rhythm [66], at least in part. As a possible mechanism of how our personalized light intervention could decrease fragmented activity rhythm of AD, it might be because such intervention could lead to circadian alignment presumably by circadian phase advances. Although it is natural to verify changes of DLMO after our personalized light intervention, we could not further assess DLMO after light intervention due to limited tolerability of our AD patients.
On the other hand, it would be hard to rule out that our finding showing decreased IV of LER at T1 may result from a consequence of regularity in their wake times obtained over consecutive two weeks of light intervention rather than light intervention itself.
Association of the RAR/LER with cognitive function
Several previous studies have reported an enhancement of cognitive function in AD patients mainly based on MMSE measurements after light intervention [24,67,68]. We further explored whether the IV of the RAR, which showed meaningful changes in response to our personalized light intervention, could reflect changes in the MMSE score. However, we found no relevance (Figure 1).
Until recently, there have been no direct evidence of those relationships. Although the MMSE is the most widely used screening instrument for AD, it is questionable whether MMSE is useful in determining the effectiveness of light intervention on cognition. Nevertheless, this study was meaningful in that we investigated the effect of light intervention on cognition in relation to the change of RAR.
Our study has some limitations. First, ambient light exposure throughout 24 hours was not controlled for our personalized light intervention, which would exert impact on our outcomes as a confounding factor [5,32]. Second, we did not demonstrate the efficacy of personalized light intervention on RAR or LER in relation with changes of endogenous circadian rhythms since the DLMO assessment after light intervention could not be done due to limited tolerability of our AD patients. Third, the limited power imposed by the modest sample size in our study may have played a role in the lack of statistical significance in most outcomes. A power analysis revealed that the number of 28 for each group is required to obtain statistical power at the recommended 0.80 level.
Furthermore, cognitive domains other than the MMSE, such as the sleep-dependent memory testing, also need to be included, verifying the effects of light intervention on cognitive function in our AD patients.
Despite these limitations, to the best of our knowledge, this is the first study that applies personalized light intervention based on the DLMO. Furthermore, this study was meaningful in that we found beneficial effects of personalized light intervention on RAR and LER in patients with mild or moderate degree of AD.
In conclusion, our findings provide evidence that personalized light intervention, regardless of blue-enriched light source, could alleviate IVs of RAR and LER in patients with mild and moderate AD, although found no evidence of significant relationships between changes in those IVs and changes in MMSE scores after light intervention. Results of study suggest that personalized light intervention could be used to improve daily patterns of activity and light exposure of AD patients.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jung Hie Lee, Seong Jae Kim, Jin Hyeong Jhoo. Data curation: Jae-Won Jang, Sun Hee Lee, In Bum Suh. Formal analysis: Seong Jae Kim, Jae-Won Jang, In Bum Suh. Funding acquisition: Jung Hie Lee. Investigation: Jae-Won Jang, Sun Hee Lee, Jin Hyeong Jhoo. Methodology: Seong Jae Kim, Jin Hyeong Jhoo. Project administration: Jung Hie Lee. Resources: Jae-Won Jang. Software: Seong Jae Kim. Supervision: Jung Hie Lee. Validation: Seong Jae Kim. Visualization: Seong Jae Kim.Writing—original draft: Seong Jae Kim. Writing—review & editing: Jung Hie Lee.
Funding Statement
This study was supported by a grant [2017R1A2B4003493] of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning, and by research funds from the Institute of Medical Science, Chosun University, Republic of Korea, 2022.
Acknowledgements
We thank AD patients and their caregivers from the Dementia Clinic at Kangwon National University Hospital in Gangwon-do, Republic of Korea.