Efficacy of Transcranial Direct Current Stimulation for Controlling of Food Craving in Subjects With Overweight or Obesity: A Pilot Study
Article information
Abstract
Objective
We aim to explore the effects of transcranial direct current stimulation (tDCS) on food craving improvement and changes in brain function associated with craving by conducting a total of 10 sessions of tDCS over a period of 2 weeks on overweight and obese subjects.
Methods
A total of 15 patients who were overweight or obese (body mass index [BMI] ≥23 kg/m2) were included. Weight, BMI, neuropsychological variables, and food craving-related variables were assessed. We measured absolute and relative power in 19 channels and analyzed quantitative electroencephalography (qEEG) according to the following frequency ranges: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–25 Hz), high beta (25–30 Hz), and gamma (30–80 Hz).
Results
After the application of tDCS, there was no significant reduction observed in weight and BMI. However, all measures related to food and eating showed a decrease in the intensity of cravings, and there was also a significant reduction in depression, anxiety, and perceived stress. In qEEG analysis, an increase in theta waves was observed in the left frontal area (F7 and F3), an increase in alpha waves in the right parietal area (P4), and a decrease in beta waves in the frontal area (FP2) and occipital area (O1).
Conclusion
This study confirmed the beneficial effects of tDCS on food craving regulation in overweight or obese individuals and observed improved scores in psychological factors such as depression and anxiety. Furthermore, neurophysiological changes related to food craving were observed using qEEG.
INTRODUCTION
Overweight and obesity are emerging as major global health problems. Prolonged states of overweight and obesity can lead to metabolic syndrome, cardiovascular diseases, and psychological issues [1]. Currently, obesity is considered a worldwide epidemic, affecting over 650 million adults globally [2]. Despite considerable attention and efforts from healthcare professionals worldwide, overweight and obesity continue to be on the rise. The primary causes of overweight and obesity stem from an imbalance between excessive energy intake and energy expenditure [3]. This imbalance is thought to result from complex interactions between behavioral, biological, and environmental factors contributing to dysregulation of energy balance [4]. Among these factors, food craving, in particular, can exacerbate such dysregulation by disregarding the homeostatic mechanisms related to appetite and nullifying the rewarding effects of food [5].
Craving is a state of intense motivation, often defined in dictionaries as a strong desire or longing. Food craving is considered a powerful urge to consume specific foods that are difficult to resist [6]. Cravings for food are commonly experienced even among regular individuals, with prevalence rates ranging from 52% to 97% in previous reports [7]. Several studies have reported associations between food cravings and future food consumption. It has been reported that individuals with higher food cravings exhibit stronger automatic approach inclinations towards food compared to those with lower food cravings [8]. Furthermore, food craving has been linked directly to body weight [6] and can potentially lead to obesity [9].
Considerable efforts have been devoted to understanding the characteristics and potential causes of food cravings. From a neurological perspective, an imbalance in the default mode network and the prefrontal cortex (PFC), which plays a crucial role in cognition and modifiable eating habits, is considered a key contributing factor to food cravings [3]. Building on this hypothesis, many scientists have sought to discover new and effective treatments to curb food cravings and combat obesity. Among them, the PFC, which is associated with inhibiting impulsive behavior for goal-directed actions, has received considerable attention in regulating food cravings [10]. Obese patients with binge-eating symptoms exhibited greater PFC dysfunction when exposed to food stimuli compared to control groups [11], and decreased activity in the right dorsolateral PFC (DLPFC) was reported to hinder appetite control, contributing to various behaviors that lead to obesity [12]. Therefore, increasing the activity of the DLPFC and regulating food cravings have been proposed as alternative approaches to obesity treatment [13].
Based on the hypothesis, non-invasive brain stimulation techniques, including transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS), have been widely used to reduce cravings, thanks to recent advancements in neuroscience [14]. Among these techniques, tDCS, which involves applying weak, safe currents of 1–2 mA for 3–20 minutes to increase cortical excitability (anodal tDCS) or decrease it (cathodal tDCS), has been reported to significantly influence cravings reduction [15]. However, in the context of eating-related symptoms, the effects have shown mixed results compared to substance addiction and craving. Most studies targeting food cravings and eating habits have used single-session right anodal/left cathodal montages with 2 mA current targeting the DLPFC. Despite testing subjects with various weight statuses and eating habits, some studies reported positive results with decreased calorie consumption and reduced food cravings [16,17]. On the other hand, other studies showed reduced food cravings but no decrease in calorie consumption [18] and some reported no change in both food cravings and calorie consumption [19].
Many studies have reported promising results, but one possible reason why the effects of tDCS could not be consistently confirmed in all studies is the limitations of assessment tools. The most consistently used domain in tDCS research is food craving, and it has typically been assessed using the Food Craving Questionnaire-State (FCQ-S) [20]. Several studies using FCQ-S have found significant effects of tDCS on food cravings, but the results have not been consistent [21,22]. FCQ-S scores have shown varying changes from 0.40% to 41.67% depending on the study conditions [23], and they have demonstrated low to moderate reliability [24]. Therefore, to evaluate the effects of tDCS on food cravings, the need for objective assessment tools to complement the food craving scale has been emphasized. Consequently, in this study, quantitative electroencephalography (qEEG) was used as an additional evaluation tool. qEEG data, which provide information on brain electrophysiological activity and serve as biological markers of brain function, can be obtained without invasive approaches such as needles or radiation exposure, and they can be acquired more quickly than other diagnostic tools. Due to these advantages, qEEG studies on treatment responses in patients with anxiety or depression disorders have been actively conducted [25].
tDCS is a prominent non-pharmacological treatment method that increases brain excitability by applying direct current stimulation to the scalp. It is known for its effectiveness in reducing symptoms of depression, anxiety disorders, as well as cravings associated with addiction. Therefore, in this study, we aim to explore the effects of tDCS on food craving improvement and changes in brain function related to craving by administering a total of 10 tDCS sessions over a period of two weeks to overweight and obese participants.
METHODS
Participants
This study was conducted from March 1, 2021, to August 31, 2022, at Daegu Catholic University Hospital and Daejeon St. Mary’s Hospital. All participants were recruited voluntarily through advertisements posted on hospital bulletin boards, as well as in the departments of psychiatry and family medicine. The inclusion criteria for the study participants were as follows: 1) age: 19 years or older and younger than 39 years, 2) body mass index (BMI) ≥23 kg/m2, and 3) voluntarily provided informed consent and cooperated with all tests and examinations according to the research protocol. The exclusion criteria were as follows: 1) individuals with a history of current or past psychiatric disorders, 2) individuals with scalp abnormalities, inflammatory reactions, or other dermatological issues that might interfere with electroencephalography (EEG) and tDCS electrode attachment, 3) individuals with other medical conditions that contraindicate the use of tDCS medical devices (e.g., presence of metal plates in the skull), 4) individuals with uncontrolled major medical or neurological diseases, 5) individuals with local neurological symptoms or signs, including seizure disorders, 6) individuals with a history of brain surgery or insertion of magnetic materials into the skull or eyes, and 7) individuals with any other medical conditions or drug use that, in the judgment of the principal investigator or investigator, would make them unsuitable for the trial. This study was approved by the institutional review board (IRB) of the Daegu Catholic University Medical Center (DCUMC IRB approval No. MDCR-19-012) and Daejeon St. Mary’s Hospital (IRB No.: DC20DIDI0017).
Detailed methods
A total of 15 patients who were overweight or obese (BMI ≥23 kg/m2) during the study period were included. The tDCS used in this study was a home medical device; therefore, the participants received user training on tDCS usage. During the user training, trained researchers helped the participants find the correct electrode placement and instructed them to take photos using their smartphones. The researchers then reviewed the accuracy of the photos taken by the participants and provided guidance for locating the correct positions based on these photos for future applications. Subsequently, mock applications were conducted using training devices while the participants were observed, and if they reproduced the correct application, they proceeded to the next step. In cases where the correct application was not reproduced, participants received retraining and were reevaluated. For participants who correctly reproduced the mock application, an instruction manual containing medical device precautions and a medical device storage kit were provided. The participants visited the clinical trial site directly at one week and two weeks after the initial application of the medical device to receive device application, and they applied the medical device at home once a day during weekdays (a total of 5 times over a week) during the research period. Through the application provided with the medical device, the actual application time and completion of the prescribed stimulation were monitored. The stimulation was set to be applied only once a day. The participants underwent efficacy and safety assessments at the baseline and two weeks after the initial medical device application.
tDCS
The tDCS device (YDS-301N) used in this study was provided by Ybrain Inc. (Seongnam, Korea). The tDCS montage involved placing the anode over the left and the cathode over the right DLPFC. To stimulate the DLPFC, the anode was positioned over F3 (according to the international EEG 10/20 system), and the cathode was placed over F4. The electrodes (6×6 cm) were attached to saline-soaked sponges and fixed onto the participants’ scalps by trained researchers. For each session, a direct current of 2.0 mA was delivered for 30 minutes, and a total of 10 sessions of tDCS were administered daily for two weeks, excluding the weekends.
Measures
In this study, the primary assessment variables included body weight and BMI before and after tDCS application at 0 weeks and 2 weeks. The secondary assessment variables, related to food craving, comprised the General-Food Craving Questionnaire-Trait (G-FCQ-T) [6], General-Food Craving Questionnaire-State (G-FCQ-S) [6], Yale Food Addiction Scale (YFAS) [26], the Dutch Eating Behavior Questionnaire (DEBQ) [27], Three-Factor Eating Questionnaire (TFEQ) [28]. Additionally, the tertiary assessment variables, related to psychological aspects, encompassed the Beck Depression Inventory-II (BDI-II), Beck Anxiety Inventory (BAI), Korean version of the Perceived Stress Scale-14 (PSS-14), The Barratt Impulsiveness Scale-11 (BIS-11), and World Health Organization Quality of Life Scale-Brief Version (WHOQOL-BREF).
Electroencephalography recording and pre-processing
This study’s methods (EEG recording, pre-processing, power spectrum analysis, and statistical analysis) were the same as the core methodology used in the authors’ previous studies [29-31]. In the past few years, the authors have studied the use of qEEG as a diagnostic marker for psychiatric disorders as described in the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), such as attention-deficit hyperactivity disorder and schizophrenia, using the same research methodology as in this study. Here, the existing EEG protocol was used to study the efficacy of the tDCS for controlling of food craving in subjects with overweight or obesity.
In the EEG measurements, a total of 19 channels from the international 10–20 system were used, including Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, P7, P3, Pz, P4, P8, O1, and O2. The measurements were taken using a 64-channel Comet digital EEG unit from Grass, Natus neurology, USA, with an ear electrode recording frequency of 800 Hz. The EEG recordings were carried out in two segments, each lasting 5 minutes. The first segment was conducted with the patient lying on a comfortable bed with their eyes open, while the second segment followed immediately with their eyes closed. During the open-eye segment, patients were instructed to focus on a “+” sign in front of them and minimize movement while trying not to think about anything specific. During the closed-eye segment, they were asked not to fall asleep. To analyze the EEG data, the fast Fourier transforms (FFT) algorithm was used for each frequency band in the selected epoch: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–25 Hz), high beta (25–30 Hz), and gamma (30–80 Hz). MATLAB 7.0.1 (MathWorks, Natick, MA, USA) and EEGLAB toolbox (https://sccn.ucsd.edu/eeglab/download.php) were employed for the analysis [32]. Prior to the analysis, several preprocessing steps were performed. The EEG data was down-sampled to 250 Hz, detrended, and the direct current component was subtracted to remove any baseline drift. Frequencies ≤1 Hz and ≥60 Hz, which might be affected by electrical noise, were filtered out. To address noise caused by blinking and muscle movement, independent component analysis was applied [33]. After the preprocessing, the corrected EEGs were visually inspected by clinical psychiatrists and EEG experts. For the analysis, artifact-free EEG readings of more than 2 minutes were selected from the five 3-minute recordings.
Statistical analyses
All values are expressed as mean and standard deviation. To evaluate tDCS effects, we analyzed all variables at the baseline and post-intervention using paired sample t-tests (including body weight, BMI, G-FCQ-T, G-FCQ-S, YFAS, DEBQ, TFEQ, BDI-II, BAI, PSS-14, BIS-11, WHOQOL-BREF, and qEEG data). The analysis was conducted using the Last Observation Carried Forward (LOCF) method, which involves replacing missing values that occur when a subject drops out before the completion of the clinical trial or at a certain time point for the efficacy evaluation variable with the last available observation from that subject. For clarity, we present topographical plots of the results of the paired sample t-tests (Figure 1). All analyses were performed using IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA), and statistical significance was set at a p-value of <0.05.
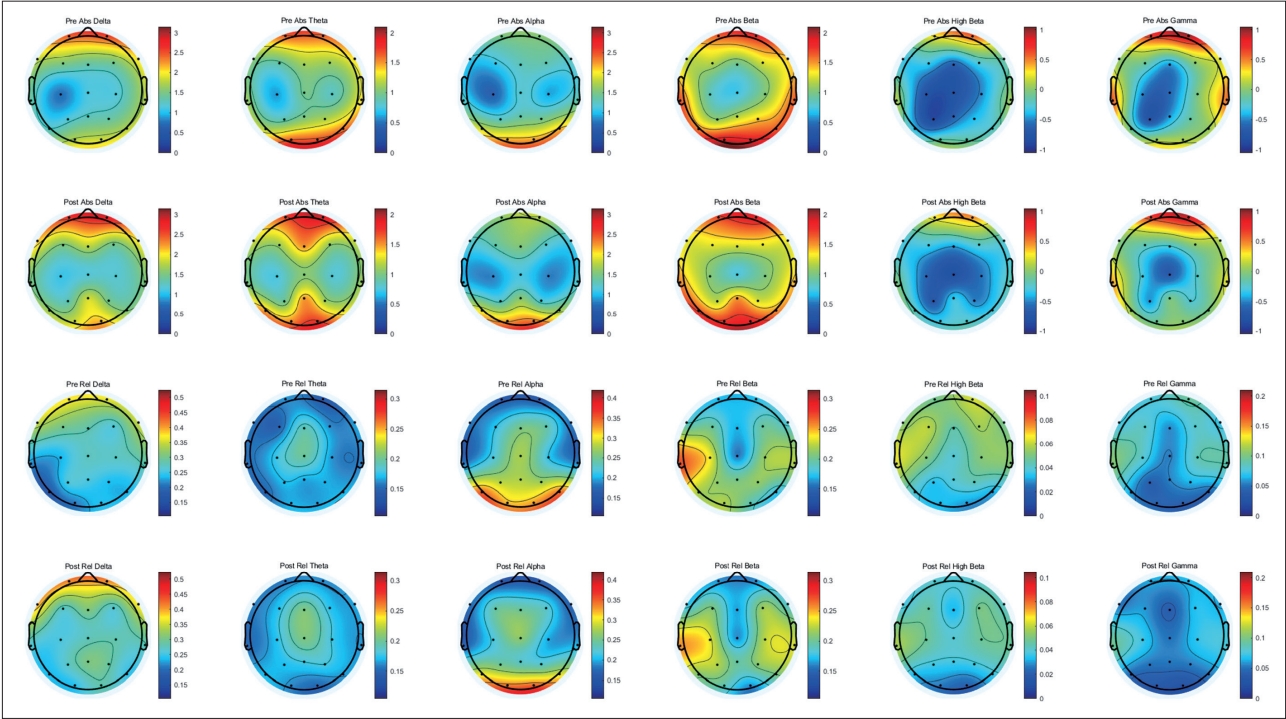
The difference in topography between pre and post tDCS intervention. Significant increase was observed in absolute theta power (F7), absolute alpha power (P4), and relative theta power (F3). Significant decrease was observed in relative high beta power (FP2) and relative beta power (O1). tDCS, transcranial direct current stimulation.
RESULTS
Demographic characteristics
The total number of participants was 15, consisting of 14 females and 1 male. All participants were overweight or obese, with a BMI of 23 kg/m2 or higher. The age of the participants ranged from 20 to 37 years, with a mean age of 31.07± 5.23 years. The average weight of the participants was 78.27±19.86 kg, and the average BMI was 29.49±5.22 kg/m². None of the participants had taken appetite suppressants in the past month, and there were no reports of psychiatric symptoms, such as depression or anxiety, during the baseline evaluation.
Weight, BMI, and psychological variables
There were no statistically significant differences in weight (p=0.575) and BMI (p=0.797) between the two conditions. However, significant differences were observed in the psychological variables. Specifically, there were significant differences in BDI-II (p<0.001), BAI (p=0.004), and PSS-14 (p=0.003) between the two conditions (Table 1).
Food craving related variables
Significant differences were observed in the G-FCQ-T total score (p=0.004). G-FCQ-T consists of four subdomains (preoccupation with food, loss of control, emotional craving, and positive outcome expectancy), and all four subdomains as well as the overall total score exhibited a significant decrease. Furthermore, a significant decrease was observed in the G-FCQ-S total score (p=0.001). G-FCQ-S comprises a five-factor structure, and all factors showed a significant decrease. Additionally, food and eating-related measures YFAS (p=0.001), DEBQ (p=0.002), and TFEQ (p=0.002) also displayed a significant decrease after tDCS intervention (Table 2).
qEEG differences between two conditions
Significant differences were observed between the two conditions in the mean absolute power and relative power for each frequency band. After tDCS intervention, there was a significant increase in absolute theta power (F7, p=0.034), absolute alpha power (P4, p=0.019), and relative theta power (F3, p=0.026). In contrast, there was a significant decrease in relative high beta power (FP2, p=0.049) and relative beta power (O1, p=0.021). No differences were observed for the delta power in any region (Figure 1).
DISCUSSION
In this study, we investigated the effects of tDCS on food craving regulation in subjects with overweight or obesity, as well as exploring psychological factors such as depression, anxiety, and neurophysiological changes like qEEG measures. The average age of the participants was 29.17 years, and all of them were female. Following two weeks of consistent tDCS application, no significant reductions were observed in weight and BMI. However, there was a notable decrease in the intensity of cravings across various food and eating-related measures, including G-FCQ-T, G-FCQ-S, YFAS, DEBQ, and TFEQ. Among the psychological variables, BDI-II, BAI, and PSS-14 showed significant reductions. Regarding qEEG measures after tDCS application, theta waves increased in the left frontal area (F7 and F3), alpha waves increased in the right parietal area (P4), and beta waves decreased in the prefrontal area (FP2) and occipital area (O1).
In conclusion, there were no significant changes in weight or BMI observed following two weeks of tDCS application. According to previous meta-analytic studies, healthy individuals with normal weight and individuals with binge eating disorders were found to benefit the most from tDCS in reducing food cravings and energy intake [34]. In this study, the participants were overweight or obese individuals without binge eating disorders, making it difficult to observe changes in weight and BMI. Many studies have shown a sustained reduction in food-related cravings and energy intake after tDCS application. These studies typically recruited participants with strong cravings for food [18,35], and in contrast, when behavioral traits were not measured or when healthy participants were primarily recruited, significant results were not observed [21,36]. Therefore, in cases where participants can already regulate their eating habits, increasing neural activity in the DLPFC may not yield significant benefits. Studies supporting this hypothesis continue to be reported, explaining that there are limitations to gaining benefits from increased cortical activity due to the ceiling effect [23].
In this study, significant reductions were observed in almost all domains of the food and eating-related measures following two weeks of tDCS application. These positive findings align with previous systematic reviews and meta-analytic studies, which reported the effects of tDCS on the DLPFC for regulating appetite, food cravings, and energy intake [34]. Our study’s positive results can be understood based on the following evidence. Abnormalities in the dopaminergic neurotransmitter system, which regulates reward sensitivity and control, have been implicated as a cause of uncontrollable food cravings and obesity, as demonstrated in previous research [37]. Based on this hypothesis, tDCS stimulation of the PFC can modulate the dopamine pathway and enhance resistance to food cravings through reinforcement of the reward system [38]. The regulation of DLPFC activity by tDCS can also alter executive functions and food reward processing in dopamine-rich regions such as the striatum and ventromedial PFC [39]. Furthermore, abnormalities in the serotonin system and low levels of brain-derived neurotrophic factor (BDNF) have been suggested as underlying causes of uncontrolled eating habits and subsequent eating disorders [40,41]. Electric stimulation like tDCS has been shown to increase synaptic serotonin activity and elevate BDNF levels, implying a positive impact on food cravings and uncontrolled eating habits. Apart from food and eating-related measures, significant reductions were also observed in BDI-II, BAI, and PSS-14. Food cravings and addiction are known to be heavily influenced by mood, impulsivity, and chronic stress [37]. Stress, cognition, and emotional processing have been found to play a more significant role in food cravings compared to other substance-related addictions [42]. Therefore, since tDCS is an appropriate treatment for mood regulation, it can be considered that participants’ food cravings decreased through this mechanism [43]. However, since mediation analysis for food and eating-related measures and psychological variables was not conducted in this study, we cannot make hasty interpretations. It is essential to explore mediation models for food cravings and psychological variables with a larger sample size in future studies.
After tDCS application, changes in qEEG were also observed. Specifically, an increase in alpha power was confirmed in the right parietal area (P4). In previous research, a study conducted neurofeedback alpha/theta (A/T) training on 50 participants, resulting in a significant reduction of intentions and plans to consume food, as well as a decrease in craving as a physiological state. A/T training was notably associated with an increase in resting EEG alpha power in several brain areas [44]. Other studies with healthy participants who underwent alpha/theta training also demonstrated a reduction in food cravings and an increase in resting-state EEG alpha activity [44]. Recently, there was a randomized controlled pilot study on the therapeutic effects of EEG Neurofeedback in adults with binge-eating disorder [45]. Adults with binge-eating disorder and overweight (n=39) were randomly assigned to a food-specific EEG neurofeedback paradigm aiming to reduce beta activity in the PFC and enhance theta activity after viewing highly appetizing food images. The EEG neurofeedback training comprised a total of 10 sessions with a 3-month follow-up period. After neurofeedback training, the participants showed significant reductions in objective binge-eating episodes, overall eating disorder psychopathology, and food cravings. Additionally, they exhibited decreased relative beta and increased relative theta power in the central frontal region [45], which is consistent with our study’s findings. Compared to obese individuals without psychiatric disorders, obese individuals with binge-eating symptoms were found to have higher resting beta power, and this elevated beta activity indicated increased perceptual and attentional biases towards specific stimuli like food [46]. Thus, recent research has set increased beta activity as an EEG indicator related to eating disorders. Theta activity is generally associated with working memory and cognitive control, and its enhancement can have positive effects on the cognition of individuals with various binge-eating symptoms [47].
The present study is not without limitations. Firstly, it is essential to mention the small sample size as the most significant limiting factor. Being a pilot study, the group size was too small to investigate group differences or detect small effects with adequate statistical power. We are currently planning to recruit additional participants, and once the sample size is achieved, we will examine the differences between the groups. Secondly, the study only included female participants. While the aim was to limit the influence of gender, for generalizability of the research findings, it would be necessary to include males in the study design. Previous meta-analytic studies have reported no significant gender differences in the therapeutic effects of tDCS on appetite, food cravings, and energy intake [34]. Thirdly, many tDCS studies on food cravings have used healthy control groups, whereas this study only analyzed the experimental group. Since the objective of this study was not only to regulate food cravings through tDCS but also to include weight loss, participants were recruited specifically as overweight or obese individuals. Lastly, the 2-week treatment period might be too short to observe significant changes in weight and BMI.
Despite the limitations, the study has notable strengths. Firstly, while most studies on food cravings and energy intake have relied on self-report questionnaires or visual analogue scales, this research additionally used objective measures such as qEEG. Such objective methods can be beneficial in investigating the effect size of craving reduction in future studies. Secondly, regarding stimulation parameters, bilateral stimulation of the DLPFC has been found to be more effective in reducing food cravings and energy intake compared to unilateral stimulation. Additionally, a multi-session protocol and an intensity of 2 mA have shown greater impact on reducing food cravings and energy intake [34]. In our study, we set the stimulation conditions known to be most effective, especially utilizing tDCS that can be applied consistently for two weeks at home. Therefore, it can be evaluated that tDCS was effectively delivered to the participants.
Conclusion
This study confirmed the beneficial effects of tDCS on food craving regulation in overweight or obese individuals and observed improved scores in psychological factors such as depression and anxiety. In addition to the commonly used self-report questionnaires, the study utilized qEEG to examine neurophysiological changes, including an increase in theta waves and a decrease in beta waves. However, no significant changes in weight and BMI were observed following two weeks of tDCS application. It is speculated that the lack of weight loss effects from tDCS in this study might be attributed to the participants not having depression, anxiety, or eating-related psychopathology. To further explore the weight loss effects of tDCS, it would be essential to recruit a larger sample size, and conduct subgroup comparisons between individuals with normal weight and those with overweight or obesity. Additionally, investigating the mediating effects of psychological factors such as depression and anxiety would be crucial.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jo-Eun Jeong, Jun Won Kim. Data Curation: Jo-Eun Jeong, Jun Won Kim. Formal analysis: Jo-Eun Jeong, Jun Won Kim. Funding acquisition: Jun Won Kim. Investigation: Jo-Eun Jeong, Jun Won Kim. Methodology: Jo-Eun Jeong. Project administration: Jo-Eun Jeong. Supervision: Jun Won Kim. Visualization: Jun Won Kim. Writing—original draft: Jo-Eun Jeong, Jun Won Kim. Writing—review & editing: Jun Won Kim.
Funding Statement
This work was supported by the grant of Research Institute of Medical Science, Daegu Catholic University (2020).


