Clonidine Patch for Tourette Syndrome With Attention-Deficit/Hyperactivity Disorder
Article information
Abstract
Objective
To explore the efficacy and safety of clonidine adhesive patch in Tourette syndrome (TS) patients with comorbid attentiondeficit/hyperactivity disorder (ADHD).
Methods
This study was conducted on a sample of children and adolescents with TS who had comorbid ADHD between May 2012 and March 2015. The patients were diagnosed according to Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, and were randomly assigned to four different dose groups: 1.0 mg/week, 1.5 mg/week, 2.0 mg/week and placebo group, and the symptom was evaluated by Swanson, Nolan, and Pelham Rating Scale, Version IV (SNAP-IV) and Yale Global Tic Severity Scale scales every 2 weeks. The primary outcome was tic disorders (TD) effective rate at week 8.
Results
One hundred and twenty-seven TS patients with comorbid ADHD in 2.0 mg/week (n=35), 1.5 mg/week (n=27), 1.0 mg/week (n=36) and placebo groups (n=29) were included in this subgroup analysis. The TD effective rate of the 2.0 mg, 1.5 mg, and 1.0 mg groups at week 8 were significantly better than that in placebo group (85.7%, 81.5%, and 86.1% vs. 20.7%, all p<0.0001). All groups demonstrated significant improvements in SNAP-IV total scale scores compared to baseline (p=0.0004), with treatment groups showing only a trend for better performance compared to placebo group at week 8, without statistical differences (22.1±15.41, 21.3±11.96, and 21.2±12.48 vs. 26.0±13.37, p=0.3385). A total of 9 adverse reactions occurred, all recovered spontaneously without additional medication.
Conclusion
Clonidine adhesive patch could safely and effectively reduce the tic symptoms of TS patients with comorbid ADHD, and might be potentially helpful in the ADHD symptoms control.
INTRODUCTION
Tourette syndrome (TS) is a type of hereditary neurodevelopmental tic disorder (TD), a complex childhood-onset neuropsychiatric disease that includes a variety of phenotypic movements and vocal tics [1]. TS often develops in association with a variety of neuropsychiatric diseases, such as attention-deficit/hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), anxiety, emotional disorder, destructive behavior, learning difficulties and sleep disorders [1,2]. The most common comorbidity was ADHD, while the second most common comorbidity in TS was OCD, diagnosed in 50% of cases of TS in the already mentioned largest service-based study to date [3]. The average prevalence of ADHD in TS patients is about 50%–60% (33%–91%), with a higher prevalence in males [4,5]. Destructive behavior and dysfunction caused by ADHD can negatively affect academic, social and family functions. Compared with TS alone, TS patients with comorbid ADHD have more defects in working memory, inhibitory function and visual attention [6]. Consequently, those patients feel a greater impact on life and learning, and therefore treatment of TS patients with comorbid ADHD has attracted much attention in recent years.
Treatment options for TS patients with comorbid ADHD include observation, the comprehensive behavioral intervention for tics, and medication [7]. First-, second-, and third-generation antipsychotic agents are all being used in TS. Haloperidol acts against dopamine D2 receptors. Risperidone acts via a dopamine D2 receptor and 5-HT2 receptor antagonism; aripiprazole reduces dopaminergic neurotransmission through D2 partial agonism; tiapride acts as a selective dopamine antagonist at dopamine D2 and D3 receptors, and so on. However, in case of TS combined with ADHD, only clonidine and guanfacine were recommended [8]. Clonidine is a selective adrenal α2 receptor agonist, which indirectly affects the central dopaminergic neurons by stimulating GABA release, thereby improving attention deficit and other hyperactivity symptoms [9,10]. It was found that oral clonidine, whether immediate release or extended release, was effective in children and adolescents with ADHD and/or TS [11,12]. Compared to psychotropic drugs, the clonidine adhesive patch has fewer adverse reactions (ADRs) than oral medications and better tolerance, reducing the number of medications [7,13].
A series of clinical studies, meta-analyses and real-world studies have shown that clonidine adhesive patch can effectively treat TD [13-15]. However, despite evidence that clonidine can effectively treat TD [16] with ADHD [17], there are only a few studies on its therapeutic effect in TD with comorbid ADHD [2,18]. A recent Cochrane study on the matter showed that clonidine could improve the symptoms, but the evidence level was low, requiring further validation studies on a larger scale [10].
Therefore, this subgroup analysis of multicenter, randomized, double-blind, placebo-controlled trial aimed to investigate the effectiveness of clonidine adhesive patch at different doses (1.0, 1.5, and 2.0 mg/week) in TS patients with comorbid ADHD.
METHODS
Study design and patients
This subgroup analysis included TS patients with comorbid ADHD from a randomized, double-blind, placebo-controlled, multicenter clinical trial. The original trial included patients with TS in 20 centers between May 2012 and March 2015 (Supplementary Table 1 in the online-only Data Supplement). And the inclusion criteria of original trial were: 1) aged between 6–18 years old, 2) body weight between 20 and 40 kg, and 3) TS diagnosed in accordance with criteria for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [19]; ADHD in accordance with DSM-IV. The exclusion criteria were: 1) patients with other disorders (for example, autism spectrum disorders or OCD) at the same time (except those with ADHD), 2) diagnosed or suspected intelligence defects, 3) obvious physical diseases (especially lower than normal blood pressure and heart disease), 4) abnormal results of laboratory or electrocardiogram examination, 5) other common diseases or drugs that may have affected the safe application of the patch, 6) allergy to clonidine adhesive patch, or severe allergic reaction to more than two drugs, 7) standardized treatment with antipsychotics, antidepressants, antimanic drugs and anticonvulsive drugs 1 week before enrollment or treatment with long-acting preparations within a service cycle, 8) treatment with any TD drugs systematically 2 weeks before enrollment, 9) treatment with clonidine adhesive patch systematically 4 weeks before enrollment, and 10) participation in clinical trials of other drugs in the last 3 months. That study was approved by ethics committee of each center (ethical approval number No. RFL201001), and registred in the National Medical Products Administration (CTR20132112). Written informed consent was obtained from the subjects (7 years or older) and their parents (legal guardians). TS patients with comorbid ADHD were selected for this subgroup analysis.
Randomization and blinding
Clonidine adhesive patch can release clonidine at a relatively constant rate for 1 week without any “trough or peak” changes in plasma concentration. Previous studies have treated TS patients with comorbid ADHD with clonidine at doses ranging from 1 to 2 mg [15]. To further explore the correlation between dose and efficacy, we set up dose groups of 1.0 mg, 1.5 mg and 2.0 mg. Patients were randomly assigned in a 1:1:1:1 ratio by block randomization to four dose groups of 1.0 mg/week, 1.5 mg/week, 2.0 mg/week, and a placebo group (0 mg/week). The random number table was generated by a statistician who was not involved in following study, using SAS 9.2 (SAS Inc., Cary, NC, USA). Both researchers and patients were blind to the grouping.
Intervention
The corresponding dose in the form of adhesive patch (manufacturer: clonidine adhesive patch, Ruifulai Pharmaceutical Co., Ltd, Taiyuan, China) was applied for 8 weeks. Application site was located under the back scapula, with replacement once every 7 days. When the old patches were replaced, the patches were folded and saved. At the end of the experiment, the researchers handed over the patches to the inspectors.
Outcomes
The demographic information (including age, sex, nation, and education) and clinical characteristics (including age of consultation, Swanson, Nolan, and Pelham Rating Scale, Version IV [SNAP-IV] score and Yale Global Tic Severity Scale [YGTSS] score at baseline) were recorded.
The primary outcome was TD effective rate at week 8, and the secondary outcomes were YGTSS score, SNAP-IV score, YGTSS and SNAP-IV reduction rates at weeks 2, 4, 6, 8, and TD effective rate at weeks 2, 4, 6. The relaiability and validity study of SNAP-IV score and YGTSS score at baseline was conducted in Chinese [20,21].
The YGTSS was used to evaluate the tic status of all children, as the quantitative assessment of the number, frequency, intensity, complexity and interference of tic symptoms and scores of motor tic and vocal tic. According to the degree of functional impairment in self-esteem, social communication, learning and work caused by tic, the overall functional impairment score (total impairment rate score) was calculated. The total score of YGTSS was obtained by adding the scores of motor tic, vocal tic and total damage rate as the efficacy evaluation index.
The ADHD status was assessed by SNAP-IV. Both parent and teacher versions of the scale had the same content and were composed of 26 items, including three subscales of attention-deficit subscale (9 items), hyperactivity/impulsivity subscale (9 items) and oppositional defiant disorder (8 items). Each question is scored on a 4-point scale from 0 to 3, indicating different levels of severity, with 0 being “not at all,” 1 being “occasionally,” 2 being “often,” and 3 being “always.” Each question with ≥2 points is used as a positive symptom criterion.
The reduction rate of each score was calculated compared to the baseline, according to the formula:
Reduction rate=(total pre-treatment score - total post-treatment score)÷total pre-treatment score×100%.
Reduction rate ≥80% was referred to as “clinical recovery”; reduction rate ≥50% and <80% as “significant improvement”; reduction rate ≥30% and <50% as “improvement” and reduction rate <30% as “ineffective.” The effective rate was calculated as percentage of cases with YGTSS reduction rate >50% compared to baseline.
The ADRs were classified as mild or grade 1, if not affecting normal daily activities; moderate or grade 2, if discomfort interfered with or reduced normal daily activities; severe or grade 3 if discomfort led to inability of performing normal daily activities.
Statistical analysis
Full analysis set (FAS) was the main set for efficacy analysis. For the case data that failed to observe the whole treatment process, the latest observation data were used to transmit to the final test result (last observation carried forward [LOCF]). Per protocol set (PPS) was the secondary analysis set for efficacy analysis and included cases in which medication did not meet the minimum criteria (70%), removed from the FAS cases and cases in which significant violations of the clinical trial protocol were found after randomization. Safety set (SS) was the primary analysis set for safety evaluation and included all randomized cases with the study drug used at least once.
SAS 9.13 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. The continuous data confirming to normal distribution was expressed as mean±standard deviation (SD), and compared using repeated measures analysis of variance (ANOVA). The categorical data was expressed as number (%) and compared using Fisher test. LOCF was used for missing data in FAS. Two-sided p<0.05 was considered as statistically significant.
RESULTS
One hundred and twenty-seven TS patients with comorbid ADHD in 2.0 mg/week (n=35, aged 9.01±1.68 years), 1.5 mg/week (n=27, aged 9.19±2.49 years), 1.0 mg/week (n=36, aged 9.23±1.93 years) and placebo groups (n=29, aged 8.82±1.77 years) were included in this subgroup analysis. Among them, 115 were male, 124 were of Han nationality, and 108 were primary school students. Sex, age of consultation, SNAP-IV and YGTSS scores at baseline were comparable among groups (all p>0.05) (Table 1).
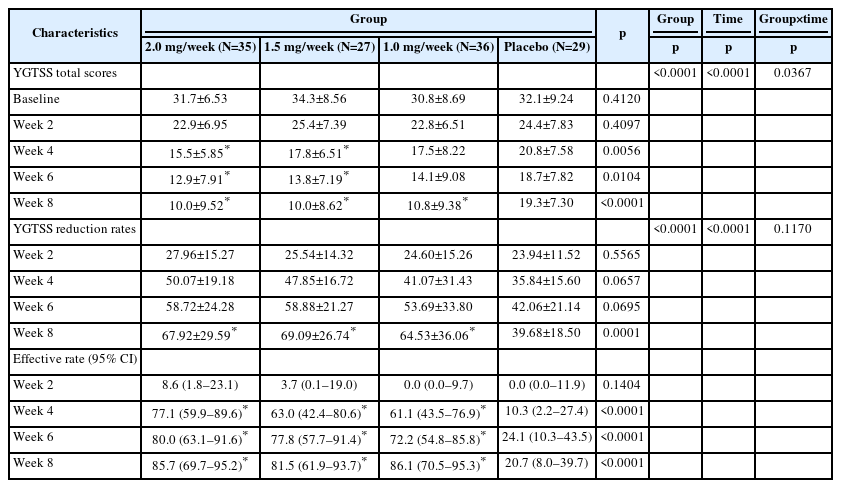
According to the LOCF analysis results of FAS, the TD effective rate of the 2.0 mg/week group (85.7% [69.7%–95.2%]), 1.5 mg/week group (81.5% [61.9%–93.7%]), 1.0 mg/week group (86.1% [70.5%–95.3%]) at week 8 were all significantly higher than that of the placebo group (20.7% [8.0%–39.7%], all p<0.001), and the effective rates among treatment groups were comparable (all p>0.05) (Table 2). After clonidine treatment, there were differences in the YGTSS score of motor tic between different dose groups and between different treatment times (p=0.0367). The greater the dose of clonidine and the longer the treatment time, the more obvious the YGTSS score of motor tic decreased. The YGTSS score of vocal tics did not differ significantly between different doses, but with the prolongation of the treatment course, the YGTSS score of vocal tics significantly decreased. The YGTSS reduction rates of the 2.0 mg/week group, 1.5 mg/week group, 1.0 mg/week group were all significantly higher than that of the placebo group at week 8 (67.92±29.59, 69.09±26.74, and 64.53±36.06 vs. 39.68±18.50, all p<0.001) (Table 2).The results obtained from the PPS analysis (Supplementary Table 2 in the online-only Data Supplement) were consistent with those from the FAS.
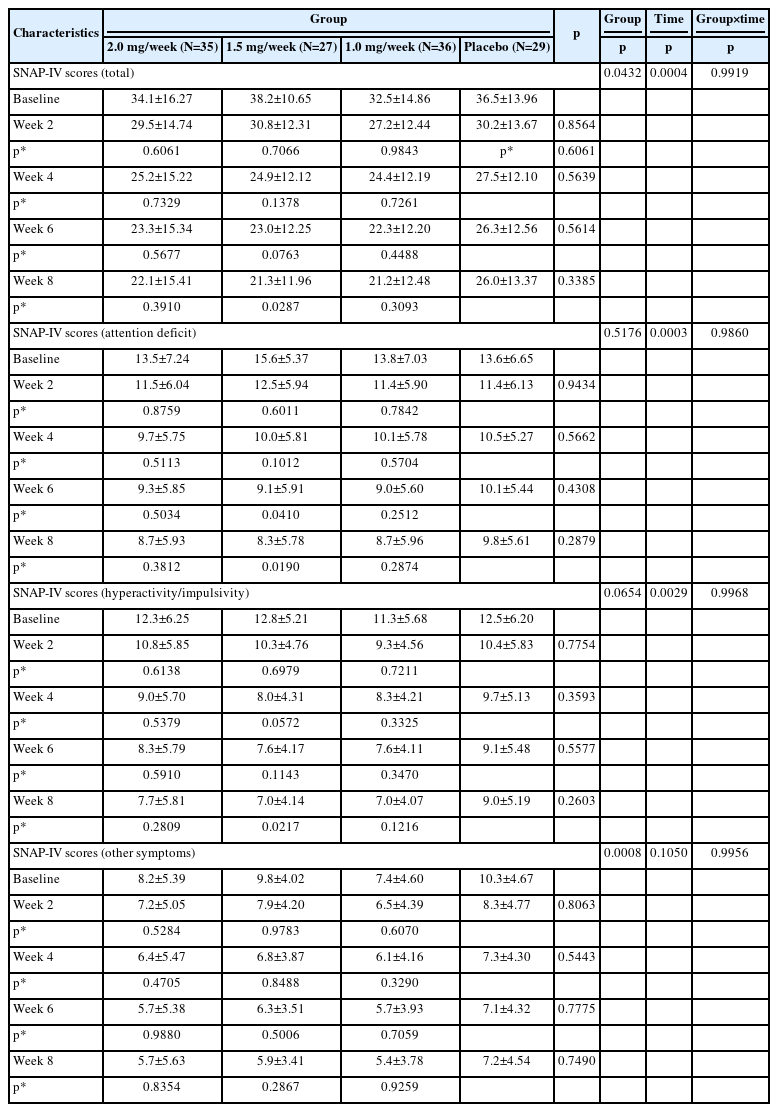
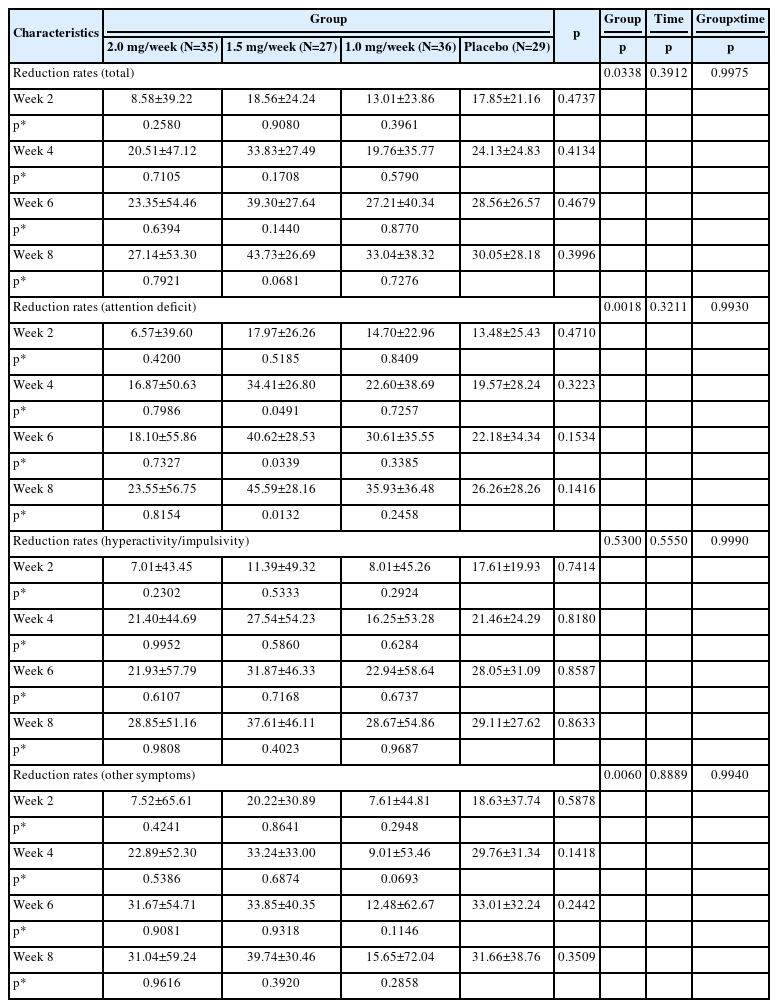
The results of FAS showed that after 8 weeks of treatment all study groups including placebo demonstrated a significant improvements in SNAP-IV total scale scores (p=0.0004), but not reduction rates (p=0.3912). There were significant differences in the total SNAP-IV scores (p=0.0432) and reduction rates (p=0.0338) among groups, but after 8 weeks of treatment SNAP-IV scores were 22.1±15.41, 21.3±11.96, 21.2±12.48, and 26.0±13.37 (Table 3), while SNAP-IV scores reduction rates were 27.14±53.30, 43.73±26.69, 33.04±38.32, and 30.05±28.18 (Table 4) in 2.0 mg/week, 1.5 mg/week, 1.0 mg/week and placebo groups respectively. Treatment groups showed a trend for better performance for most visits, but without significant difference (p=0.3385 for SNAP-IV scores, p=0.3996 for SNAP-IV scores reduction rates), most scores decreased in all groups as the number of weeks of treatment increased. And there were differences between the 1.5 mg/week group and the placebo group in total scores, scores of attention deficit and hyperactivity/impulsivity at week 8, while there were no differences between the 1.0 mg/week group and the 2.0 mg/week group compared to the placebo group.
In the PPS (Supplementary Table 3 in the online-only Data Supplement), the SNAP-IV scores of the 2.0 mg/week, 1.5 mg/week, 1.0 mg/week groups were slightly lower than that of the placebo group at week 8, but without statistical significance (24.1±15.89, 20.4±11.28, 20.9±12.41, and 26.4±13.02, p=0.2569). There were no significant differences among the four groups at every visit, and the differences between any treatment group and the placebo group in the dimension of hyperactivity/impulsivity and other symptoms were not statistically significant.
Compared to baseline, treatment groups and placebo group showed significant continual reduction in SNAP-IV total score and subscores, both in FAS (Table 4) and PPS (Supplementary Table 4 in the online-only Data Supplement). But no significant difference was found between the four groups at every visit.
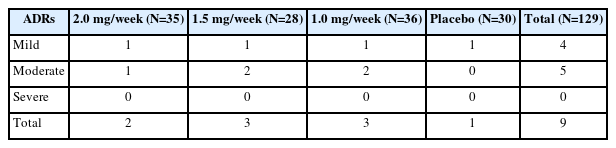
The safety evaluation was based on the SS, and included a total of 129 case, 9 ADRs (6.98%) occurred in the study, all of which were skin-related. After the ADRs, 4 cases continued the medication, 2 cases suspended the medication, and 3 cases discontinued the medication. All participants who experienced ADRs recovered spontaneously without additional treatment (Table 5, Supplementary Tables 5 and 6 [in the online-only Data Supplement]).
DISCUSSION
This study found that different doses of clonidine adhesive patch were equally effective in improving the TD symptoms and significantly reducing the YGTSS scores. Besides, treatment groups only slightly differed from the placebo in the symptomatic improvement of ADHD during the visits but without significant difference. Those findings support the view that clonidine adhesive patch can significantly improve tic symptoms in TS, while potentially aiding in the ADHD symptoms control.
The course of TS in children has its distinctive characteristics, and although ADHD symptoms usually improve during adolescence, in some patients TS with comorbid ADHD may persist into adulthood [6,22]. It was previously shown that combination of TS and ADHD is more often reported in males, with Hirschtritt et al. [23] study demonstrating the probability of TS with comorbid ADHD in male and female patients being respectively 58.5% and 42.3% (p<0.01). This study included TS patients with comorbid ADHD, and among 127 participants male to female ratio was 9.5:1, notably higher compared to Hirschtritt et al. [23] study. Although the prevalence of males is consistent with previous reports, recent studies draw attention to the fact that this broad discrepancy is at least partly due to the lack of recognition and referral bias in females [24]. In some countries including China the above bias might be even more pronounced [25], as demonstrated by this study results, and must be taken into account while planning the individualized intervention strategies.
For the treatment of TS with comorbid ADHD, current European guidelines recommend clonidine and guanfacine as first-line treatment for mild to moderate TD [8], while Canadian [26] and Chinese [27] guidelines recommend only clonidine as the first-line treatment for TS with comorbid ADHD. A recent meta-analysis that included six randomized, placebo-controlled trials showed a wide range of doses for α-2 agonists used for successfully reducing tic and ADHD symptoms, with clonidine dosages ranging from 0.175 orally to 1.5 in a form of skin patch [28]. In an earlier study by Yang et al. [13] patients with weight 20–40 kg were given dosage of 1.0 mg; 40–60 kg were given 1.5 mg, and >60 kg were given 2.0 mg – all those dosages were reported to be effective compared to the tiapride. The results of a systematic review by Wang et al. [15] also discussed the dosage ranges from 1.0 mg to 2.0 mg as being effective and safe. This study found that the use of clonidine adhesive patches significantly reduced YGTSS scores in TS patients with comorbid ADHD, which is consistent with the results of previous studies. All dosages were effective compared to the baseline, but did not significantly outperform the placebo in the SNAP-IV score improvement. The reason for not observing statistically significant differences for treatment groups may be due to the insufficient sample size, however, both FAS and PPS showed slightly better reduction rates in the attention deficit dimension in the 1.5 mg/week group. Although there was no significant difference among groups, 1.5 mg/week showed a trend to better performance compared to other groups at week 4, week 6 and week 8, suggesting that this dose should be closer investigated in the future as the most appropriate dosage for this age group. The specific pathophysiological mechanisms may require further clarification in future research.
Clonidine was an α-adrenergic agonist, and it was found that noradrenergic fibers project from the blue-spot to the cerebral cortex and modulate frontal subcortical circuits associated with TS. Stimulation of central α-2 adrenergic receptors leads to reduced catecholamine release through GABA-regulated feedback [29]. This may be the mechanism of clonidine acts on the α-2 receptor in TS.
In the safety analysis, only 6.98% of patients had ADRs, similar to the findings of Yang study [13], where the incidence of ADRs was 7.1%, and lower than the incidence of ADRs for the oral TD drugs [10,30]. In this study, the ADRs were all linked to rash, mild to moderate, without new serious safety concerns. Only one case in each dose group discontinued treatment due to ADRs, thus the incidence of ADRs was not significantly different in the dose groups, suggesting that proposed treatment is safe in all dosages.
This study has several limitations. The number of female participants was very small, which may result in the lower value of the study findings for the female population. As it was a subgroup analysis, some of the differences are not statistically significant, and require a larger sample size for confirmation. And finally, although children in different groups had comparable age of consultation, some potential differences such as body mass index were not taken into account. Nevertheless, our study provides important insights into the management of TS with comorbid ADHD.
In conclusion, clonidine adhesive patch could safely and effectively reduce the tic symptoms in TS patients with ADHD, and might be potentially helpful in the ADHD symptoms control.
Supplementary materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2023.0262.
Centers included in this study
YGTSS improvements in the PPS
SNAP-IV scale improvements in the PPS
Summary of SNAP-IV scale reduction rates in the PPS
Detailed ADRs cases analysis (SS)
Comorbid ADHD ADRs aggregate (SS)
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Yasong Du. Formal analysis: Yanhui Chen, Zhongling Ke. Investigation: Yanhui Chen, Zhongling Ke. Methodology: Yanhui Chen, Ying Ouyang, Ying Han, Dong Liang, Xueping Gao, Jie He, Yasong Du. Resources: Jie He. Supervision: Yanhui Chen, Ying Ouyang, Ying Han, Dong Liang, Xueping Gao, Jie He, Yasong Du. Writing—original draft: Zhongling Ke. Writing—review & editing: Yanhui Chen.
Funding Statement
None
Acknowledgements
None