Effects of Serious Games in Older Adults With Mild Cognitive Impairment
Article information
Abstract
Objective
The rising prevalence of mild cognitive impairment (MCI) has spurred interest in innovative cognitive rehabilitation approaches, including serious games. This review summarizes randomized clinical trials (RCTs) exploring the impact of serious games on MCI patients.
Methods
We conducted a comprehensive data search using key terms such as “gamification,” “digital therapy,” “cognition,” “mild cognitive impairment,” and “Alzheimer’s disease.” We exclusively considered published RCTs, excluding animal studies and basic research.
Results
We identified eight RCTs. Four RCTs examined the effects of serious games using cognitive training for MCI patients. Notably, one study found that non-specific training (Nintendo Wii) significantly enhanced cognitive function and quality of life compared to cognition-specific computer training (CoTras). Among the remaining three RCTs, one specifically demonstrated that personalized serious game-based cognitive training yielded superior cognitive outcomes and reduced depressive symptoms. One RCT focused on serious games incorporating physical exercise, highlighting the effectiveness of kinetic-based exergaming in enhancing overall cognition. Three RCT focused on combined cognitive training and physical exercise. A double-blind RCT revealed that progressive resistance training or standalone physical exercise outperformed the combined approach in improving executive function and global cognition. Two additional RCTs reported positive outcomes, including improvements in cognitive function and electroencephalogram patterns associated with game-based interventions.
Conclusion
Serious games, whether focusing on cognitive training, physical exercise, or a combination of both, have potential to improve cognitive and functional outcomes in individuals with MCI. Further research and standardization of protocols are needed to better understand the full potential of serious games in MCI.
INTRODUCTION
In the face of a rapidly aging global population, the prevalence of mild cognitive impairment (MCI) has become a growing concern [1]. MCI, often considered an intermediate stage between normal age-related cognitive decline and more severe conditions such as Alzheimer’s disease (AD), represents a critical point in the trajectory of cognitive health in older adults [2]. As individuals with MCI grapple with cognitive challenges that hinder their daily lives, researchers and healthcare professionals have sought innovative interventions to not only alleviate cognitive symptoms but also to enhance cognitive function and improve quality of life (QOL) [3].
The newly the United States Food and Drug Administration (FDA)-approved drug, lecanemab, has shown potential in slowing disease progression in patients with MCI and early AD by removing amyloid-β deposition in the brain [4]. However, there are concerns about the high risk of side effects associated with this drug, including brain edema and hemorrhage, also known as amyloid-related imaging abnormalities [5]. Furthermore, it is important to note that while the newly FDA-approved drug may slow disease progression, it does not have any effect on improving cognitive decline [6]. Therefore, the development of new therapy for patients in the trajectory of AD or MCI remains a critical area of research.
One innovative approach is the utilization of serious games, which is a growing field within digital therapy. These games have shown promise in addressing cognitive deficits among older adults with MCI [7]. Serious games are defined as games primarily designed for learning and education, rather than entertainment, making them an exciting avenue for cognitive intervention [8,9]. These digital games, often accessible through a range of electronic devices, offer a unique and engaging platform to stimulate cognitive processes, promote social interaction, and provide a sense of accomplishment [10].
Numerous prior studies have investigated the effectiveness of serious games in improving cognitive abilities. However, these studies were conducted in diverse subjects, ranging from subjective cognitive decline, MCI, to dementia, with diverse etiologies, including stroke, Parkinson’s disease, fronto-temporal dementia, and AD [11-13]. In addition, studies utilized diverse designs to assess the effects of serious games in clinical settings. The targets of serious games also varied, from improving cognitive functions to enhancing physical abilities or to decreasing behavior psychological symptoms including activities of daily living (ADL) and depressive symptoms. Collecting and condensing the results of these investigations is vital to make informed judgments regarding the efficacy of serious games in older adults with MCI.
This review paper aims to summarize randomized clinical trials (RCTs) that investigated the effect of serious games on both cognitive and non-cognitive functions in patients with MCI. By shedding light on the therapeutic potential of serious games, this review aspires to contribute to a greater awareness of the importance of embracing innovative approaches in the pursuit of cognitive health and a better QOL for older adults with MCI.
DATA SEARCH
We used the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, Medline, PsycINFO, PubMed, the Web of Science using the key words “gamification,” “digital therapy,” ”cognition,” “mild cognitive impairment,” and “Alzheimer’s disease” to identify published articles. We excluded studies which were conducted in patients with dementia. We only included RCTs conducted in human, so animal studies and basic research were not included. The language was restricted to English. The data search was initially conducted on July 1st, 2023, with the second and validation search conducted on August 1st, 2023.
S.M.W. and S.H.K initially reviewed the abstracts identified from the literature search independently to determine whether they met the scope of our review. Two other authors (D.W.K. and Y.H.U.) re-evaluated the papers to confirm whether they clearly met the selection criteria. If a disagreement occurred, the article in question was discussed and a consensus was reached through the review of the three remaining authors (C.U.L, S.Y.L, and H.K.L.). This article is a narrative review focusing on the clinical trial investigating effect of serious games on people with MCI. All relevant studies meeting the scope of the present paper were selected based on the consensus among the authors.
RESULTS
The search resulted in eight RCTs that investigated the effects of serious games on individuals with MCI. The studies were very heterogenous in their design, settings, subjects, and outcome measures. Consequently, we categorized them into following groups: 1) studies that included serious games using cognitive training only (refer to Table 1), 2) studies that included serious games with physical exercise only (refer to Table 2), and 3) studies that involved serious games with a combination of cognitive training and physical exercise (refer to Table 2).
Serious games using cognitive training for patients with MCI
We identified four clinical trials investigating the effect of serious games using cognitive training only (Table 1). Among these four RCTs one was single-blinded (SB), and the other three were open label (OL) studies. The SB, or rater blinded, RCT was conducted in Korea by Park and Park [14]. A total of 79 subjects with MCI were randomly assigned to receive either cognition-specific computer training using CoTras (Netblue, Daegu, Korea) (n=39) or non-specific computer training using Nintendo Wii (Nintendo Co., Kyoto, Japan) (n=39). Both groups received 30-minute intervention three times a week for 10 weeks. Interestingly, after 10 weeks of treatment, the Wechsler Adult Intelligence Scale (WAIS) subtests (including digit span forward: 0.48±0.08 vs. 0.12±0.04; digit span backward: 0.46±0.09 vs. 0.11±0.04) and health related QOL (vitality: 9.05±1.17 vs. 2.69±1.67; role-emotional: 8.31±1.20 vs. 4.15±0.71; mental health: 11.62±1.63 vs. 6.95± 1.75; bodily pain: 4.21±2.17 vs. 0.10±0.38) were significantly higher in the non-specific computer training group than in the cognition-specific computer training group (p<0.05). The authors postulated that the non-specific computer training requires continued effort to adapt to new tasks, which could enhance attention and other cognitive functions. Non-specific computer training might have facilitated the training in a more enjoyable manner using an avatar and a virtual reality (VR) environment. By doing so, non-specific computer training might have also led to higher motivation, resulting in better adherence than cognition-specific computer training.
Among the three OL-RCTs, the first study was conducted in the US. The study by Gooding et al. [15] included a total of 96 patients with subclinical cognitive decline. Subclinical cognitive decline was defined as 1) subjective or informant memory complaints, 2) verbal memory impairment, defined as >0.5 standard deviation on Wechsler Memory Scale-Revised Logical Memory II or Buschke Selective Reminding Test (BSRT), 3) Mini Mental State Examination (MMSE) score >24, and 4) normal ADL. Thus, the inclusion criteria closely resembled early-stage amnestic MCI. Patients were randomized into three groups: the computerized cognitive training group (CTG), the cognitive vitality training group (CVG), or the control group (CG). Participants in the CTG (n=31) engaged in Posit Science’s BrainFitness (Posit Science, San Francisco, CA, USA), a game based cognitive training program designed to improve memory, attention, and executive functions. Those allocated to the CVG (n=23) underwent the same cognitive training using BrainFitness as CTG but were allowed to modify BrainFitness with the three components of Neuropsychological and Educational Approach to Remediation (NEAR) model of treatment (i.e., personalization, autonomy, and contextualization) [16]. The goal of the NEAR-based manipulation was to promote independence, treatment engagement, intrinsic motivation, and self-efficacy. Subjects in the CG received various commercially available computer games and puzzles (e.g., BrainAge, Sudoku, crossword puzzles). All groups received the same intensity of treatment, 60 minutes per session, twice a week for 16 weeks. The results showed that the three groups did not differ in baseline demographic data including age, neuropsychological measures, and depression severity (Beck Depression Inventory-second edition [BDI-II]). Regarding the change scores of neuropsychological measures, both experimental groups (CTG and CVG) showed greater preservation of functioning on a measure of global cognition in modified MMSE than the CG. In addition, the CTG group performed better than the CG on one measure of verbal learning (BSRT total score) and both measures of verbal memory (BSRT delay T-score and logical memory subtest). Similarly, CVG showed superior outcome than the CG on one measure of verbal learning (BSRT total score) and one measure of verbal memory (logical memory subtest). Lastly, CTG and CVG showed no group difference in cognitive function measures. In terms of emotional functioning, CVG demonstrated a relative decrease in depressive symptoms (BDI-II) after treatment compared to the CG group, who showed a relative increase in depressive symptoms. No significant differences were observed between the CVG and CTG groups or between the CTG and CG. In summary, both CVG and CTG groups performed superior to CG in cognitive measures, with better depression symptoms observed only in the CVG. Although between-group comparisons did not reveal significant differences between the CVG and CTG groups, participants enrolled in the CVG demonstrated the largest treatment gains suggesting importance of personalized approach in game based cognitive training.
In the second OL-RCT, Savulich et al. [17] investigated the effects of game-based cognitive training on cognitive functions in patients with MCI. Forty-two subjects were randomized to the treatment group (TG) (n=21) or CG (n=21). The TG received one hour per session of an iPAD-based memory game, twice a week for 4 weeks, while the patients in the CG received usual care only. The study demonstrated significant time-by-pattern-by-group interactions for cognitive performance, including the number of errors made and trials needed on the Cambridge Neuropsychological Test Automated Battery (CANTAB) Paired Associates Learning task (p=0.044; p=0.027), CANTAB Paired Associates Learning first trial memory score (p=0.002), MMSE (p=0.036), the Brief Visuospatial Memory Test (p=0.032), and the Apathy Evaluation Scale (p=0.026). In addition, the TG maintained higher levels of enjoyment and motivation to continue after each hour of gameplay, with selfconfidence and self-rated memory ability improving over time.
The last OL-RCT was conducted in Korea. Thapa et al. [18] studied the effects of VR-based cognitive training in older adults with MCI. A total of 68 patients with MCI were randomized to the TG (n=34) and the CG (n=34). The patients in the TG underwent a total of 24 sessions, three times per week, and with each session lasting 100 minutes, involving VR-based cognitive training for 8 weeks. The VR-based cognitive training included activities such as Juice making, Crow shooting, finding the Fireworks number, and memorizing object in the house. In addition, the TG received an educational program regarding general health care led by health professionals, an exercise specialist, a physical therapist, and a nutritionist once a week for eight weeks. Patients in the CG received the same educational program but without the VR-based cognitive training. Analysis of the subjects for group–time interactions showed that the TG exhibited a significantly better mean change in gait speed, 8-feet up and go, trail making test-B (TMT-B), and symbol digit substitution test (SDST). Previous electroencephalogram (EEG) research has shown that a higher Theta/Beta ratio (TBR) is related to mind wandering and reduced attention [19]. Similarly, Thapa et al. [18] showed that the TBR was decreased in the temporal (p=0.035) and parietal (p=0.027) regions at follow up for the TG, whereas the CG did not show any changes. Thus, the authors suggested that the EEG test results could reflect a positive change in brain activity related to attention after VR-based treatment.
Serious games using physical exercise for patients with MCI
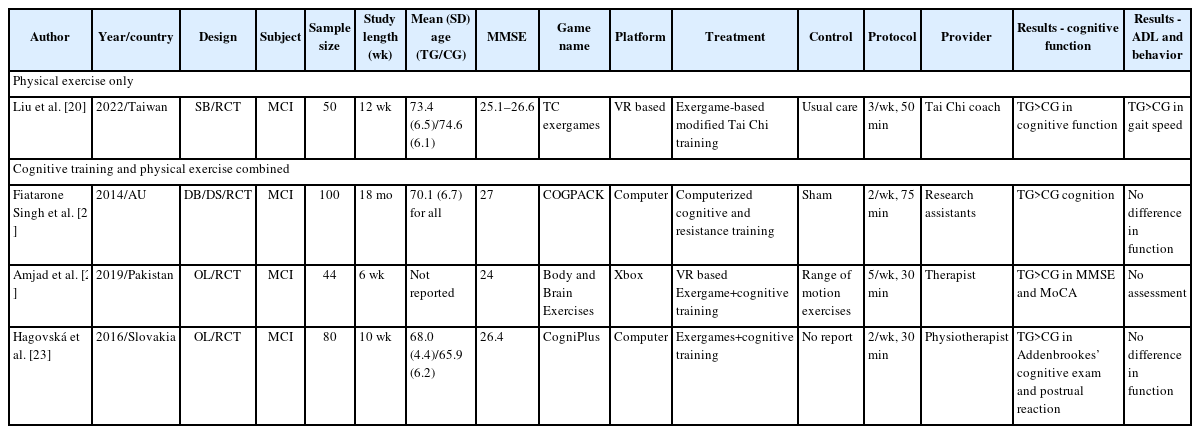
We identified one clinical trial that investigated the effects of serious games using physical exercise in patients with MCI (Table 2). In a study conducted in Taiwan, Liu et al. [20] investigated the effects of exergaming-based Tai Chi on cognitive function, dual-task cost, and gait performance compared with traditional Tai Chi and CGs in older adults with MCI. A total of 50 patients with MCI were randomly assigned to a VR based Tai Chi exergame group (n=16), a traditional Tai Chi group (n=17), or a CG (n=17). Both VR-based and traditional Tai Chi groups received 36 training sessions (three, 50-minute sessions per week) over a 12-week period, while the CG received no intervention and were instructed to maintain their usual daily physical activities. There were no significant between-group differences in baseline age and MMSE. The results showed that after 12 weeks of treatment, both VR-based and traditional Tai Chi groups performed better than the CG in the trail making test-A (TMT-A), TMT-B, Delta TMT, Stroop Color and Word Test (SCWT) seconds, and one-back test. In addition, only the VR-based Tai Chi group, but not the traditional Tai Chi group, had a significantly higher mean score for the MoCA (Montreal Cognitive Assessment) and SCWT number compared with the CG (MoCA: p=0.008; SCWT number: p=0.001). In terms of gait performances, both VR-based and traditional Tai Chi groups performed better than the CG in gait speed and dual-task cost of speed during cognitive dual-tasks after training (gait speed: exergaming-based Tai Chi [EXER-TC] vs. control, p<0.001, traditional Tai Chi [TC] vs. control, p=0.001; dual-task cost of speed: EXER-TC vs. control, p=0.002, TC vs. control, p=0.017). Thus, the study suggested that VR-based Tai Chi was at least comparable to, if not superior to, the traditional Tai Chi in improving cognitive and physical function in patients with MCI.
Serious games with combined cognitive training and physical exercise for patients with MCI
Three clinical trials investigating the effect of serious games with combined cognitive training and physical exercise in patients with MCI were identified (Table 2). The first study by Fiatarone Singh et al. [21], conducted in Australia, was the only double blind (DB)-RCT. Patients with MCI, aged 55 or above, were randomized into four groups: cognitive training+sham exercise (n=24), progressive resistance training (n=22)+sham cognitive training, combined cognitive intervention and progressive resistance training (n=27), and a CG which received both sham cognitive training and sham exercise (n=27). The cognitive training involved computer-based multimodal and multidomain exercises targeting memory, executive function, attention, and speed of information processing using COGPACK (Marker Software, Ladenburg, Germany) program. Progressive resistance training included high-intensity exercise led by experienced research assistants, including exercise physiologists and physiotherapists. Sham cognitive consisted of watching 5 short National Geographic videos, followed by a set of 15 questions (3/video) regarding the presented material. Lastly, sham exercise involved light stretching and seated calisthenics with no effect in heart rate, balance improvement, or strength enhancement. All patients received intervention 2–3 days per week for 6 months with an 18-month follow-up. The results showed no group differences in the median training duration of 26 weeks, and dropout rate was low (8% and 12% at 6 and 18 months, respectively). The Alzheimer’s Disease Assessment Scale-Cognitive Subscale was significantly improved in the progressive resistance training group compared with sham exercise (p<0.05) over 6 months, with a trend for benefit persisting over 18 months (p=0.08). The executive function test (WAIS Matrices) was also improved in progressive resistance training group compared with sham exercise (p<0.02) at 6 months. However, there was no difference between cognitive training and sham cognitive for both cognition and executive functions. In terms of four-group analysis, contrary to the authors’ hypothesis, the combined group did not perform better than the single intervention arms for both cognition and executive functions. In fact, the progressive resistance training+sham CTG showed the highest efficacy for executive function and the global domain at 18 months followed by cognitive training+sham exercise group. The combined group either performed last (executive functions) or second last (global domain). There were six adverse musculoskeletal events over 18 months (three falls during assessments and three exacerbations of pre-existing arthritis symptoms during strength testing/training, with one unresolved (exacerbation of an underlying rotator cuff tear).
The second study was an OL-RCT that investigated the effects of Xbox 360 Kinect cognitive games (XBox, Redmond, WA, USA) on the slowness and complexity of EEG, as well as cognitive functions in patients with MCI [22]. A total of 44 MCI subjects were randomized into the TG (n=22, Xbox 360 Kinect cognitive games) and the CG (n=22, range of motion exercises) for 30 minutes per session, 5 times a week, over a 6-week period. The results showed that in the TG, MMSE (26.25±0.347 vs. 23.722±0.731, p=0.003), MoCA (25.65±0.310 vs. 22.00±0.504, p=0.0001), TMT-A (1.429±0.234 vs. 2.225±0.259, p=0.028), and TMT-B (2.393±0.201 vs. 3.780±0.195, p=0.0001) improved significantly at 6 weeks compared to the baseline. In addition, delta (0.673±0.029, p=0.013), theta (0.129±0.013, p=0.002), beta2 waves (0.044±0.009, p=0.046), and the complexity of EEG (0.051±0.042, p=0.016) were also improved at 6 weeks compared to the baseline in the TG. However, these changes were not observed in the CG.
The third study, also an OL-RCT, compared game-based cognitive training with dynamic balance training (TG) and balance training session alone (CG) in cognitive functions, postural control, and functional status in patients MCI. A total of 80 subjects with MCI were divided into the TG (n=40) and the CG (n=40) [23]. The TG received cognitive training twice per week, 30 minutes per session, for 10 weeks using CogniPlus (SCHUHFRIED, Mödling, Austria) together with balance training. The CG received a balance training program for the same duration and frequency. The TG performed better in cognitive function, Addenbrooke’s cognitive examination (p<0.05–0.0001), and Balance Evaluation Systems Test (p<0.05–0.0001) than the CG. However, no significant differences were noted in the evaluation of functional activities.
DISCUSSION
The purpose of this review was to provide a narrative summary of published articles investigating the effects of serious games on cognitive function, ADL, and emotional symptoms in patients with MCI. Our review included a total of eight research articles. Among them, four RCTs investigated the effect of serious games using cognitive training, one RCT utilized serious games incorporating physical exercise, and finally, three RCTs employed serious games combining both cognitive training and physical exercise. Regarding the study design, only one was a DB-RCT, two were SB, and five were OL. Across all studies, there was consistent result indicating that serious games demonstrated superior efficacy compared to usual therapy or conventional treatments in improving cognitive function, ADL, and/or emotional symptoms.
In terms serious games using cognitive training, one Korean study compared cognition-specific computer training (CoTras) to non-specific computer training (Nintendo Wii) [14]. The non-specific training group exhibited significant improvements compared to the cognition-specific computer training in cognitive function and QOL. The mechanism behind the nonspecific computer training showing higher efficacy over the cognition-specific computer training is still unclear. Cognitive function can be improved by enhancing the ability to learn new tasks [24]. Moreover, higher motivation is reported to be associated with greater compliance and better intervention outcomes [25,26]. In this study, the non-specific computer training group was required to continuously adapt to new tasks, potentially having positive effects on attention and leading to better cognitive functions. Similarly, the avatar and VR environment might have facilitated the training and increased the patients’ motivation in an enjoyable manner. Consistent with these results, a previous study showed that patients with Parkinson’s disease trained with Wii for 4 weeks performed better in attention (95% confidence interval: -1.49 to -0.11) than those trained with the cognition-specific computer training (CogniPlus) [27]. However, further translational studies are needed to understand the biological mechanism underpinning the difference between the cognition-specific and non-specific computer training in the human brain.
Three OL RCTs from the US and Korea explored the effects of serious games using cognitive training [15,17,18]. All three RCTs showed positive outcome in cognition. Game based-cognitive training, especially when personalized, showed better cognitive outcomes and reduced depressive symptoms compared to the non-personalized serious game based-cognitive training [15]. Another study indicated that those who received game-based cognitive training, in contrast to those who received usual care, exhibited higher levels of enjoyment and motivation to continue after each hour of gameplay, with self-confidence and self-rated memory ability improving over time [17]. The study might suggest the importance of motivation and self-confidence when patients are provided with cognitive training. The last RCT revealed an interesting result: game based-cognitive training not only enhanced the cognitive function but also improved physical abilities, including gait speed [18]. The study utilized VR-based games requiring physical motion (e.g., crow shooting). Although these games were not designed to improve physical functions, the motions associated with the game might have had a positive effect on physical coordination and psychomotor speed.
In terms of serious games with physical exercises, one RCT was conducted in Taiwan [20]. The study compared VR-based Tai Chi exergaming to traditional Tai Chi, and the results showed that both interventions significantly improved cognitive and physical functions compared to the CG [20]. Further studies are needed to explore whether game-based physical exercise yields better cognitive outcome than conventional physical exercise.
Three RCTs explored the effects of serious games combining cognitive training and physical exercise. In an Australian DB-RCT, which had the longest study length of 18 months, progressive resistance training was found to be the most effective in improving executive function and global cognition in patients with MCI [21]. Contrary to the authors’ hypothesis, the combination of cognitive intervention with progressive resistance training showed the least benefit. The exact reason for this result is not clear; however, even the CG received both sham cognitive training and sham exercise. Sham cognitive training consisted of watching five short National Geographic videos, followed by a set of 15 questions (3/video) regarding the presented material. The sham exercise consisted of light stretching and seated calisthenics, with no impact on heart rate, balance improvement, or strength enhancement. These sham treatments might have had a positive effect on the patient’s cognitive function, potentially resulting in a high placebo or sham effect.
The second RCT, an OL study from Pakistan, showed that Xbox 360 Kinect cognitive games reported significant improvements in cognitive function compare to those who received range of motion exercises or the CG [22]. In addition, the complexity of EEG was improved only in the Xbox 360 Kinect group. Thus, the study suggests that cognitive improvement from serious games combining cognitive training and physical exercise might be associated with enhanced EEG complexity. The last RCT, also an OL, conducted in Slovakia, demonstrated that game-based balance training combined with cognitive training led to better cognitive and postural control than those who received conventional balance training [23].
Overall, these trials demonstrate that serious games, whether focusing on cognitive training, physical exercise, or a combination of both, have the potential to improve cognitive and functional outcomes in individuals with MCI. Personalization and adaptability in game-based interventions appear to be key factors for success. However, further research and standardization of protocols are needed to better understand the full potential of serious games in managing MCI.
Notes
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Conflicts of Interest
Hyun Kook Lim, a contributing the Editor-in-Chief and Yoo Hyun Um, a contributing editor of the Psychiatry Investigation, were not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Sheng-Min Wang, Hyun Kook Lim. Data curation: Sheng-Min Wang, Hyun Kook Lim. Formal analysis: Sheng-Min Wang, Hyun Kook Lim. Funding acquisition: Hyun Kook Lim. Investigation: Yoo Hyun Um, Dong Woo Kang, Soyoung Lee. Methodology: Sheng-Min Wang, Hyun Kook Lim, Sunghwan Kim. Project Administration: Yoo Hyun Um, Dong Woo Kang, Chang Uk Lee. Resources: Sheng-Min Wang, Sunghwan Kim. Software: Sheng-Min Wang, Sunghwan Kim. Supervision: Soyoung Lee, Chang Uk Lee. Validation: Yoo Hyun Um, Dong Woo Kang, Soyoung Lee. Visualization: Chang Uk Lee, Yoo Hyun Um. Writing—original draft: Sheng-Min Wang. Writing—review & editing: Sheng-Min Wang, Hyun Kook Lim.
Funding Statement
This research was supported by Culture, Sports, and Tourism R&D Program through the Korean Creative Content Agency grant funded by the Ministry of Culture, Sports, and Tourism in 2022 (Project number: R2022020030, contribution 100%).
Acknowledgements
None