Association Between Hypnotics and Dementia: A Mini Narrative Review
Article information
Abstract
Objective
This narrative review aims to provide a comprehensive assessment of the existing literature on the relationship between hypnotics and dementia, considering both potential link and inconclusive or lack of association.
Methods
Data from studies that investigate the association between hypnotic medications and dementia were reviewed. Studies included both cohort studies and systematic reviews, participants with various type of dementia and hypnotics including benzodiazepines (BZDs) and Z-drugs (ZDs).
Results
The existing literatures presents conflicting evidence regarding the association between hypnotics, including BZDs and ZDs, and the risk of dementia. Some studies suggest a potential link between prolonged use of hypnotics and an increased risk of dementia. However, other studies indicate inconclusive or lacking evidence regarding this association. Factors such as study design, sample characteristics, and control of confounding variables contribute to the variability in findings.
Conclusion
The relationship between hypnotics and dementia remains complex and controversial. While some studies suggest a potential association, others find inconclusive or conflicting evidence. Future research should focus on addressing methodological limitations, considering classifying dementia subtypes, and try to adjust medication lag time.
INTRODUCTION
Sleep is a fundamental component of overall health and well-being. For example, sleep health can affect cardiovascular disease, obesity, psychiatric disease, and dementia [1]. For this reason, difficulty in sleep can significantly comprises public health including psychiatric diseases, posing challenges for a substantial portion of the population [2]. In response to these sleep-related issues, the widespread use of hypnotics, especially benzodiazepines (BZDs), and Z-drug (ZD), has emerged as a common treatment [3]. Nevertheless, ongoing debates persist regarding the potential long-term consequences of prolonged hypnotics use on health [4-6].
In 2019, an approximate 57.4 million individuals across the globe were afflicted by dementia, and prognostications indicated that the incidence of such cases was poised to nearly triple within the ensuing three decades [7]. Modifying several risk factors, like hypertension, obesity, diabetes mellitus and smoking, could help prevent or delay up to 40% of dementias [8]. Recent years have witnessed a mounting body of research investigating the nexus between hypnotics and dementia, sparking heightened interest in potential mechanisms involved [9-12]. Concerns have been raised about the use of certain hypnotics, notably BZDs and ZD, due to their well-documented central nervous system inhibitory effects [13,14]. Especially, the effect of hypnotics on cognitive function has prompted further investigation into their potential association with dementia.
The possible correlation between the use of hypnotics and the dementia has been a topic of significant research and controversy. On one hand, studies have suggested a potential link between prolonged use of hypnotics and an increased risk of dementia [9,10]. Conversely, skeptics maintain that the evidence remains inconclusive and that other factors may account for the observed correlations. Some studies have failed to establish a clear causal connection between the use of hypnotics and dementia [11,12].
In this narrative review, we will examine the arguments and findings on both sides of controversy. We aim to provide a balanced assessment of the existing literature on the relationship between hypnotics and dementia, considering the strengths and weaknesses of each perspective. Additionally, we will delve into the limitations of current research within this domain and address unresolved inquiries, guiding future directions for investigation. Through these efforts, we endeavor to not only enhance the contemporary comprehension of the interplay between hypnotics and dementia but also to inform evidence-based decision-making in clinical practice and public health.
POTENTIAL LINK BETWEEN HYPNOTICS AND DEMENTIA
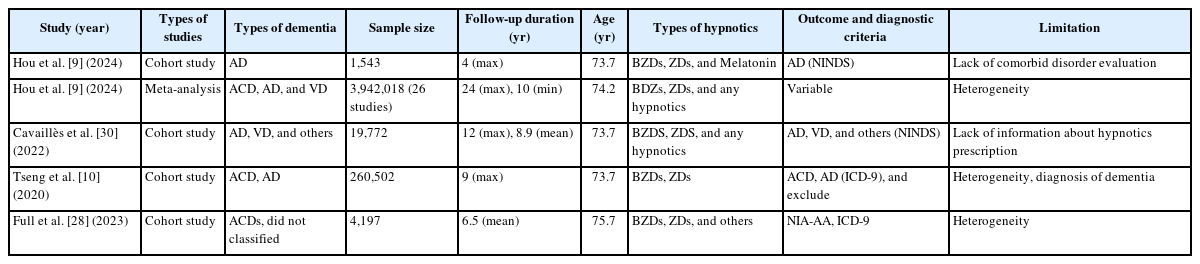
Table 1 presents a summary of research investigating the potential link between hypnotic medications and dementia. BZDs are frequently prescribed psychotropic medications used to treat conditions such as anxiety and insomnia, while ZDs are another class of drugs commonly prescribed, particularly among older adults, to manage insomnia [15]. From a pharmacological perspective, BZDs function as positive allosteric modulators of gamma-aminobutyric acid (GABA)-A receptor in the central nervous system, leading to hypnotic, anxiolytic, and muscle-relaxant properties [16]. ZDs produce clinical effects similar to those of BZDs, but they differ from BZDs in that they specifically bind to the alpha-1 subunit of the GABA-A receptor [17]. BZDs and ZDs are not recommended for long-term use due to their adverse effect, especially cognitive decline, psychomotor retardation, drug tolerance and dependency [18-21]. Particularly when it comes to cognitive decline, there is a common belief that the prolonged use of these medications heightens the likelihood of developing dementia.
When researchers investigate dementia, it is crucial to differentiate between specific causes of dementia, such as Alzheimer’s disease (AD), vascular dementia (VD), and others. These conditions are distinct diseases with varying causes and pathological processes, which can significantly impact the diversity of the studied population [22]. The biological and pathological characteristics of different types of dementia exhibit unique features specific to each condition. AD is characterized by the presence of neuritic plaques containing amyloid beta (Aβ) and neurofibrillary tangles containing phosphorylated tau [23]. VD, the second most common type of dementia, may result from vascular diseases including small chronic infarcts, multiple microinfarcts, and large infarcts [24]. Other forms of dementia, such as those due to alpha-synucleinopathy and frontotemporal dementia, have their own distinct pathologies, such as alpha-synuclein [25] and phosphorylated TDP-43, MAPT, or FUS proteins [26]. While each type of dementia has its unique pathophysiology, certain clinical aspects may overlap among various etiologies [27].
Numerous studies exploring the link between hypnotics and dementia did not categorize dementia based on its etiologies but found the association between hypnotics and all-cause dementia [9,10,28]. In one meta-analysis of 26 longitudinal studies, they found a correlation that depended on the dosage, showing a connection between the use of hypnotic medications and the risk of developing dementia [9]. As for the subtypes of dementia, the relationships were significant in all cause dementia, but not in VD. 9 The findings suggested that the risk of developing dementia increased by 5% in individuals who used the daily recommended dose for 100 days, relative to non-users [9]. In one study in Taiwan, researcher’s also analyzed 260,502 participants who aged older than 65 years with who were prescribed hypnotics include BZDs and ZDs [10]. In this study, BZDs were categorized into short-acting and long-acting BZDs based on their half-life, and they identified all cause dementia event except VD [10]. As a result, individuals who used short-acting BZDs and ZDs were found to be at a 3.25-fold higher risk of developing dementia compared to those who used long-acting BZDs [10]. Additionally, their findings indicated that individuals who used multiple hypnotic medications had a greater risk of dementia compared to those who used only one of these drugs. 10 In this study, researchers also hypothesized that BZD use might reduce the expression of GABA-A receptors in synapses, potentially leading to a decrease in postsynaptic inhibition [29]. Furthermore, they proposed that BZD use could impact GABA-A receptor-mediated synaptic currents in hippocampal cornu ammonis area 1 (CA1) pyramidal neurons, potentially disrupting the balance of excitation and inhibition in the central nervous system [29]. In United States, study was conducted in communities who had high risk of atherosclerosis, including BZDs, non-BZD receptor agonists (ZDs), or other hypnotics were categorized as hypnotics users [28]. In this cohort, hypnotics use was associated with a 48% greater risk of dementia compared to non-use, when adjusted age, sex, depression [28]. These findings are consistent with previous stance that hypnotics increase the risk of dementia.
Conversely, certain studies have revealed a correlation between AD or VD and hypnotics use, identifying them as potential risk factors [9,30]. One recent study proposed that the utilization of BZDs and ZDs was linked to an elevated risk of AD in a sizable cohort [9]. In this study, 1,543 participants were assessed to determine the association between hypnotics including BZDs and ZDs, and AD. The findings suggested a dose-dependent relationship between hypnotic use and the risk of AD, 1–5 years duration hazard ratio (HR) is 1.97, over 5 years exposure duration HR is 2.26 [9]. They suggested that because of GABA-A receptor which increased chloride conductance by BZDs and ZDs [31], so it resulted in synaptic inhibition in central nervous system [32]. In another study, researchers examined the association between excessive daytime sleepiness, complaints of insomnia, the use of hypnotic medications, and the risk of dementia in a cohort of 6,851 individuals aged 65 or older in France, none of whom had been diagnosed with AD, VD and others at the study’s baseline [30]. They suggested that the use of hypnotics, particularly BZDs was independently linked to an elevated risk of all-cause dementia, especially AD but not in VD, even after further adjustment of depressive status. In this study, researchers described the correlation between the use of BZD and AD on the focus of Aβ accumulation, a known contributor to the development of AD [30]. They proposed that chronic use of BZDs by patients could potentially impair the glymphatic system, responsible for transporting cerebrospinal fluid and removing metabolic waste from the brain. Such impairment may lead to the accumulation and deposition of Aβ in the brain [33].
INCONCLUSIVE OR LACK OF ASSOCIATION BETWEEN HYPNOTICS AND DEMENTIA
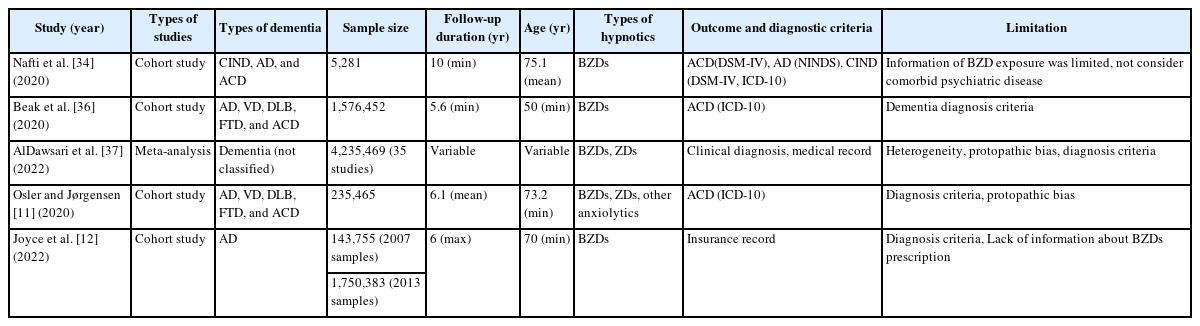
Table 2 summarized the literature found inconclusive or lack of association between hypnotics and dementia. Contrary to the perspective of the aforementioned studies, there were also literatures that question the relationship between hypnotics and dementia. In a Canadian study, conducted with 5,281 participants aged 65 years and older [34]. It was observed that current usage of BZDs was linked to a heightened risk of cognitive impairment that did not progress to dementia (HR=1.36; 95% confidence interval=1.08–1.72), but this association was not found in cases of AD or all cause dementia [34]. In this paper, the researchers suggested that they did not evaluate the frequency and dose of BZDs and not evaluate the comorbid diseases could affect results such as anxiety, but suggested that it was consistent with previous studies [35] that BZD did not increase the risk of AD. Another nationwide study in Korea also found that no association was observed with BZDs use compared with the antidepressant use [36]. In their study, following the initial 5 years of BZD exposure, the risk of dementia was found to be 23% higher in BZD users compared to non-users, but in longer lag-time, the increased HR of dementia associated with BZD use decreased [36]. The researchers suggested that the positive correlation observed between BZDs and dementia in the shorter lag-time period could potentially be attributed to protopathic bias [36]. They also suggested that the positive association between hypnotics and risk of the dementia reported in previous studies may have been confounded by depression [36]. In one meta-analysis, they evaluated 35 studies about hypnotics and dementia [37]. It was found that none of the examined hypnotic medications exhibited a link to an elevated risk of dementia, except for BZDs and ZDs [37]. Additionally, after adjusted age and protopathic bias, there were no evidence that BZDs and ZDs increased risk of dementia [37]. Moreover, in a study conducted using a nationwide cohort with total 235,465 participants in the Danish National Patient Registry, no significant association was detected between the utilization of hypnotics and the risk of dementia [11]. In the same study, it was observed that patients with the lowest utilization of BZDS or ZDs had a slightly higher odds ratio for dementia, whereas those with the highest usage had the lowest likelihood of dementia development [11]. In another case-control study, researchers examined the association between AD and BZDs in two separate samples, one from medical insurance groups from 2006 to 2012, and another from groups covering the period from 2013 to 2020 [12]. In order to minimize any bias resulting from pre-diagnosis use of medication, they compared rates of AD and related dementia diagnosis for beneficiaries exposed and unexposed to BZDs for cervical/lumbar pain, stenosis, and others, none of which are associated with dementia [12]. After that, incident BZDs use was not linked to an increased risk of dementia. In other study, patients prescribed concurrent antidepressant and hypnotic treatment, antidepressants alone, and hypnotics alone, all of them were at an increased risk of developing all-cause dementia. However, in a subgroup with depression, sleep disorders and anxiety disorders, negative association was detected [38]. These results indicated inconsistent results oppose the findings regarding positive association between the hypnotics and dementia as discussed. In previous studies, it was suggested that BZDs could reduce brain amyloid deposition for these results [39]. As mentioned above, BZDs decreases neuronal activity through GABA-A receptor, and researchers of studies above suggested that the reduction of neuronal activity could reduce axonal dystrophy and synaptic loss [40]. They also explained that other possible hypothesis are related to the effect of BZDs on translocator protein (TSPO) 18 kDa, which was formerly known as peripheral BZD receptor [40]. Specifically, the effect of BZDs on amyloid as ligands of TSPO may be mediated by modulation of neuroinflammation via the synthesis of neurosteroids, which has been found to modulate amyloid pathology [40].
LIMITATION AND FUTURE CONSIDERATIONS FOR THE RESEARCH ON HYPNOTICS AND DEMENTIA
There are several limitations to the previous research on the relationship between hypnotics and dementia risk, which we must be taken into consideration. Tables 1 and 2 summarized the limitation of past studies. First, heterogeneous sample and methodology adopted may have challenged the demonstration of a causal relationship between hypnotics and dementia risk [41]. Due to the nature of longitudinal studies on dementia, many researchers have opted to utilize databases from healthcare systems such as the National Health Service. While this approach may introduce heterogeneity among participants, researchers may choose these methods due to ethical considerations and the cost-time efficiency [42]. Although many studies have been conducted, the follow-up period, age, and diagnosis criteria of dementia in the cohort were too diverse, so in systematic review, it could be affects the evidence level rated ‘very low’ [9]. Also, uncontrolled covariates like psychiatric disorders including anxiety disorders, depressive disorder, substance use disorder and sleep disorder can influence the association between hypnotics and dementia, because they could serve as either risk factors or early signs of dementia [43]. Second, protopathic bias, which hinder the establishment of definite causal conclusions [37,41,44]. Protopathic bias, also known as reverse-causal bias, can pose a significant challenge in observational studies where data is collected over an extended period. This bias arises from the possibility of reverse causation, where the observed association between variables may be influenced by the outcome preceding the exposure [45,46]. When medications are prescribed to alleviate prodromal symptoms of the disease under investigation, it can introduce bias into studies by artificially increasing the number of cases in the group with prior use of the drug [46]. This could potentially lead to an overestimation of the association between the drug and the risk of the disease. To address this protopathic bias in studies, one approach is to implement a lag time, which involves excluding exposure for a specified period before the diagnosis [47]. In studies evaluating the association between hypnotics and dementia, researchers employ lag times to control for protopathic bias, but this alone could be difficult to completely exclude primary bias, which can affect inconsistent results. BZDs and other hypnotics are often prescribed for the symptoms had association with dementia, including depression, insomnia and anxiety [46,48]. This could lead to a protopathic bias, especially in research with short follow-up durations. Third, many associations studies adopted all-cause dementia risk as an outcome variable. Nonetheless, distinct pathological mechanisms are implicated in various subtypes of dementia, such as AD, VD, dementia due to Parkinson’s disease or Lewy body disease, and dementia due to frontotemporal degeneration. For example, diagnosis of AD involves a biomarker-based classification [49], but outcome variables included in previous studies preclude biomarkers including amyloid positron emission tomography, plasma, and cerebrospinal fluid biomarkers. Additionally, stage of cognitive impairment should be considered with more stringent classification of participants. For examples, one study revealed that BZD usage had a correlation with mild cognitive impairment, not AD [34]. In this research, the investigators proposed that the use of BZDs might exacerbate the clinical manifestations of cognitive impairment no dementia rather than demonstrating the onset of AD [34]. Finally, individual mechanisms of the hypnotics should be considered. In recent study, suvorexant, the dual receptor antagonist of orexin, which is a wake-promoting neuropeptide recently known to be associated with AD pathology, acutely decreased tau phosphorylation [50]. Considering a diverse receptor profile involved in each hypnotics and its association will be critical in future research on the association between hypnotics and dementia.
Despite the various limitations mentioned above, there are still many conflicting opinions on the relationship between hypnotics and dementia. Future studies should be performed with stringent recruitment process with structured design. Also, to find specific mechanisms about cognitive decline associated with hypnotics, homogeneous sample with the same dementia pathology should be recruited, and biomarker profiles should be considered to truly reflect the pathophysiological link between hypnotics and dementia. Finally, larger prospective cohort studies with extended follow-up durations will be necessary to establish a causal relationship.
Notes
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Conflicts of Interest
Yoo Hyun Um, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Yoo Hyun Um. Data curation: all authors. Investigation: all authors. Methodology: all authors. Project administration: Sung-Hoon Yoon. Supervision: Yoo Hyun Um. Validation: Yoo Hyun Um. Visualization: Sung-Hoon Yoon. Writing—original draft: Sung-Hoon Yoon. Writing—review & editing: all authors.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1I1A1A01057792).
Acknowledgements
None