Methamphetamine Use Disorder and Inflammation: A Case-Control Study
Article information
Abstract
Objective
Methamphetamine use disorder (MUD) is a global health condition that impairs a person’s health which may result in morbidity and mortality. Inflammation is a crucial process playing a vital role in MUD. For this reason, it is necessary to examine biochemical parameters for follow-up and treatment alternatives.
Methods
We aimed to reveal the relationship between inflammatory response and MUD by evaluating peripheral hemogram parameters, leukocyte count, subtypes, and their ratios to each other, systemic immune inflammation index (SII), monocyte/high-density lipoprotein (HDL) ratio, and human C-reactive protein (CRP) in adult men with MUD. We included 76 adult male participants in the patient group and 70 adult male participants in the control group. We calculated the neutrophil/lymphocyte rate (NLR), monocyte/lymphocyte rate (MLR), platelet/lymphocyte rate (PLR), and basophil/lymphocyte rate (BLR). In addition, we obtained the SII and the monocyte/HDL rate.
Results
The patients’ leukocyte (p<0.001), platelet (p<0.001), plateletcrit (PCT) (p=0.002), neutrophil (p<0.001), monocyte (p=0.002), CRP (p<0.001), NLR (p=0.001), PLR (p=0.004), MLR (p=0.009), SII (p<0.001) and monocyte/HDL ratio (p<0.001) were higher than the control group. We observed a significant and positive relationship between the daily methamphetamine intake, and methamphetamine use duration (p=0.002), PCT (p=0.044), neutrophil (p=0.021), NLR (p=0.001), PLR (p=0.004), MLR (p=0.029), and SII (p<0.001). Daily methamphetamine intake had a significant and positive effect on SII. A one-unit increase in daily methamphetamine intake elevated SII by 165.53 units.
Conclusion
The results confirm the presence of peripheral subclinical inflammation and systemic immune inflammation in adult men with MUD.
INTRODUCTION
The Diagnostic and Statistical Manual of Mental Disorders, fifth Edition (DSM-5) defines substance-related and addictive disorders in two sub-categories: substance use disorders (SUD) and substance-induced disorders. SUD is a chronic and recurrent disorder characterized by increased intake of legal and illicit drugs despite adverse consequences [1,2]. Methamphetamine is a potent central nervous system psychostimulant that affects the brain’s neurotransmitter system. It is highly addictive and neurotoxic to the central nervous system [2,3]. Methamphetamine, also known as “ice” or “crystal,” has seen a steady increase in abuse in recent years, with more than 33 million people worldwide [3].
Methamphetamine use disorder (MUD) is a health emergency for which no effective treatment exists. It has a high prevalence of associated mental and physical health problems [4]. Depression, schizophrenia, Parkinson’s disease, psychosis, and other neuropsychiatric disorders are more likely to develop in methamphetamine abuse, which may be primarily due to methamphetamine-induced neurotoxicity. The mechanisms underlying this neurotoxicity can be oxidative stress, excitotoxicity, and neuroinflammation [3]. Methamphetamine abuse is related to infectious diseases (HIV, hepatitis, etc.), periodontal disease, cerebrovascular events, pulmonary hypertension, stroke, kidney failure, and an increased risk of infection [3]. Methamphetamine devastates host immunity by enhancing the risk of pathogen transmission and exacerbating disease progression [5].
MUD can cause anxiety and depression. In addition, attention, memory, and cognitive problems may occur even during the remission period. Several varieties, including C-reactive protein (CRP), intercellular adhesion molecule-1, interleukin-8, interleukin-23, and vascular endothelial growth factor, were shown to be significantly associated with neuropsychiatric outcomes [4]. Exposure to methamphetamine affects both central and peripheral immune system functions, including changes in B and T cell expression, macrophage function, and natural killer cell activation [4]. Methamphetamine-administered mice represented increased macrophage, neutrophil, and proinflammatory cytokine values but low T lymphocyte levels [6].
Studies about psychiatric disease etiologies have focused on inflammation and inflammatory parameters. Immune system changes and subclinical inflammation accompany most psychopathologies [7]. The role of subclinical inflammation in mental illnesses is still unclear. Inflammation is a physiological mechanism acting in tissue injury recovery and infection elimination. However, abnormal responses or chronic inflammation may be pathological [8]. The inflammatory mediators are linked to many psychiatric disorders and contribute to neuroinflammation [3,7-9].
Studies linking inflammation with SUD are available in the literature. Blood biomarker studies are one of the most used methods. However, many of these biomarkers are difficult to collect or expensive to measure routinely, which may limit the practical application in clinic. Platelet (Plt) count, mean platelet volume (MPV), plateletcrit (PCT), white blood cell count, subtypes, and their ratios to each other (neutrophil/lymphocyte rate [NLR], monocyte/lymphocyte rate [MLR], platelet/lymphocyte rate [PLR], basophil/lymphocyte rate [BLR], systemic immune inflammation index [SII], etc.) are inexpensive determinants of inflammation which can be measured easily by a complete blood count [8-14]. CRP is an acute phase reactant produced by the liver and is involved in the inflammatory response. It is used as a biomarker of systemic inflammation in clinical practice [15]. Macrophages and monocytes are vital in synthesizing and releasing proinflammatory and prooxidant cytokines. High-density lipoprotein (HDL) preserves the endothelium from the harmful effects of low-density lipoprotein (LDL) and prevents LDL oxidation. HDL thus exhibits anti-oxidant and anti-inflammatory activities. The monocyte/HDL ratio may be a potential inflammatory and oxidative stress parameter [16]. SII is a much more critical marker in showing inflammation and immune response than PLR and NLR. It is calculated using Plt, neutrophil, and lymphocyte values (SII=Plt×neutrophil/lymphocyte). High SII is related to several diseases’ severity and poor prognosis [11,14,17].
There are studies examining PLR and NLR in patients with methamphetamine, heroin, cocaine, cannabis, and synthetic cannabinoid use disorders [9,10,18,19]. On the other hand, MPV, PCT, MLR, BLR, monocyte/HDL ratio, and inflammatory markers such as SII were not evaluated to confirm the presence of subclinical inflammation in MUD. Overall, limited information is available other than the short-term effects of MUD. Examining the potential role of inflammation in the MUD pathophysiology may help us understand how methamphetamine influences the immune system in the long term, open new avenues of intervention involving the inflammatory system in treatment, and inform and guide public policy.
This study aims to reveal the relationship between inflammatory response and MUD by evaluating routine biochemistry and peripheral hemogram parameters, monocyte/HDL ratio, NLR, PLR, MLR, BLR, MPV, PCT, SII, and CRP in individuals with MUD.
METHODS
Research design
In this prospective study, we included patients admitted to Erzurum Regional Training and Research Hospital Alcohol and Drug Addiction Research Treatment and Training Center diagnosed with MUD between October 1, 2022, and December 31, 2022. The study was carried out following the Helsinki Declaration. Erzurum Regional Training and Research Hospital Ethics Committee approved the study (2022/16-154).
Research sample
The sample consisted of 99 patients diagnosed with MUD. The study excluded 13 patients who used other substances alongside methamphetamine and 6 patients with an acute or chronic disease. The remaining 80 subjects were composed of 76 male and 4 female patients. Female patients were also excluded from the study since the number of female patients was low, which would adversely affect the statistical results. 76 male patients were included in the study. The patients had used methamphetamine for at least 1 year and tested positive for methamphetamine in the urine toxicology test on the first day of hematological measurement. Since smoking is common in people with SUD, a control sample group was formed according to the patient’s smoking status.
The patient group comprised 76 adult male patients who met the DSM-5 criteria for MUD, did not use drugs other than methamphetamine simultaneously, and had no other mental health problems. The control group comprised 70 adult male participants who applied to our outpatient clinic for consultation or status reports and met the inclusion and exclusion criteria. An experienced psychiatrist evaluated all participants. The clinical examination and the structured clinical interview for the DSM-5-clinician version (SCID-5-CV) were used to diagnose MUD and exclude additional psychopathologies.
Inclusion criteria for the patient group
To be diagnosed with MUD according to DSM-5 diagnostic criteria; 18–65 years of age; no obesity (body mass index [BMI] <30 kg/m2); no additional psychopathology in terms of psychiatry; and no acute, chronic, or autoimmune/inflammatory disease; providing written and verbal consent to participate in the study; not having any physical or mental disability preventing completion of the tests; and not using antibiotics, probiotics, corticosteroids, or other immunomodulators in the 3 months before sampling.
Inclusion criteria for the control group
18–65 years of age; no obesity (BMI <30 kg/m2); no additional psychopathology in terms of psychiatry; and no acute, chronic, or autoimmune/inflammatory disease; providing written and verbal consent to participate in the study; not having any physical or mental disability preventing completion of the tests; and not using antibiotics, probiotics, corticosteroids, or other immunomodulators in the 3 months before sampling. Smoking and non-addictive alcohol intake were not accepted as exclusion criteria for both groups. All participant records were reviewed based on clinical examinations and laboratory tests to assess their physical health. Participants’ clinical and sociodemographic information was collected. As a result, the study sample consisted of 146 adult males, 76 in the patient group and 70 in the control group.
Data collection tools
Sociodemographic-clinical data form
The researcher prepared the form, including information about the patient and control groups’ age, education level, marital status, job status, alcohol and cigarette use, height, weight, and BMI.
SCID-5-CV
The structured clinical interview for DSM (SCID) is one of the most used diagnostic tools in clinical trials worldwide. Its latest version is SCID-5. The SCID-5-clinician version (SCID-5/CV) is a standardized tool for assessing major psychiatric disorders based on DSM-5 definitions and criteria. SCID-5/CV has 17 diagnostic categories with only exploratory questions and 32 with detailed diagnostic criteria [20]. A validity and reliability study of the Turkish version of SCID-5/CV was conducted [21].
Blood samples
We checked the vital signs and performed new physical examinations to assess the presence of inflammatory disease in all participants before blood collection. We used the samples taken routinely after fasting for 12 hours in the outpatient follow-up.
Biochemistry and complete blood count measurement
The blood analyses were carried out in Erzurum Regional Training and Research Hospital Biochemistry Central Laboratory using an automatic hematological analyzer (Sysmex XN-1000, SYSMEX, Japan) and a biochemistry (Atellica, SIEMENS, Germany) analyzer.
Standard reference ranges for biochemistry and hemogram: leukocyte 4.39–11.59×109/L, neutrophil 1.8–6.98×109/L, lymphocyte 1.21–3.77×109/L, monocyte 0.29–0.95×109/L, basophil 0.01–0.07×109/L, Plt 152–383×109/L, MPV 9.3–12.1 fL, PCT 0.17%–0.32%, HDL 40–999 mg/dL, and CRP 0–5 mg/L.
We obtained NLR, PLR, MLR, and BLR values from the hemogram results by dividing the absolute numbers of the relevant cells by the lymphocyte number. We calculated SII with the formula (Plt×neutrophil/lymphocyte). We assessed the monocyte/HDL ratio by dividing the number of monocytes by HDL.
Statistical analysis
We carried out the analyses with the IBM SPSS 20 (IBR Corp., Armonk, NY, USA) statistical analysis program. We presented the data as standard deviation, mean, minimum, median, maximum, percentage, and number. We evaluated the normal distribution of continuous variables with the Shapiro–Wilk W test, Kolmogorov–Smirnov test, Q–Q plot, skewness, and kurtosis. In comparing two independent groups, we used the Independent Samples t-test when the normal distribution condition was met and the Mann–Whitney U test when it was not. We calculated the effect sizes between the two groups using Cohen’s d statistics.
In 2×2 comparisons between categorical variables, we used the Pearson chi-square test if the expected value was >5 and the Fisher’s Exact test if the predicted value was <5. For comparisons greater than 2×2 between categorical variables, we used the Pearson chi-square test when the expected value was >5 and the Fisher-Freeman-Halton test when the predicted value was <5. Correlation analysis and linear regression analysis were performed to evaluate the relationship between quantitative variables. If there was a normal distribution, Pearson correlation compared two quantitative variables. The Spearman correlation test was used if a normal distribution did not occur. We acknowledged that the statistical significance level was p<0.05.
RESULTS
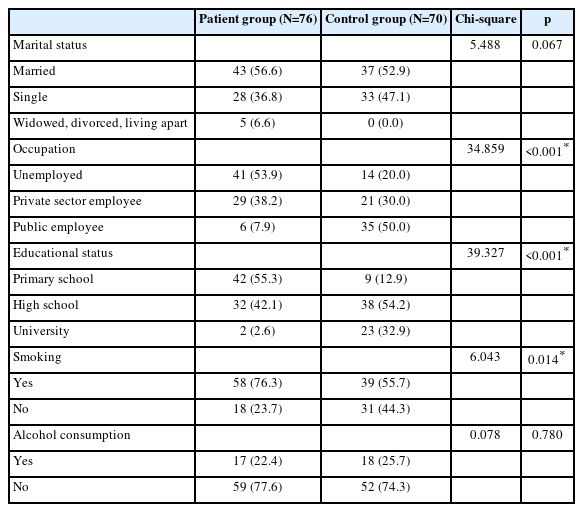
53.9% of the patients were unemployed. There was a significant difference between the patient and control groups regarding education level (p<0.001). Smoking was significantly higher in the patient group compared to the control group (p=0.014). The comparison of the sociodemographic characteristics of the patient and control groups is shown in Table 1.
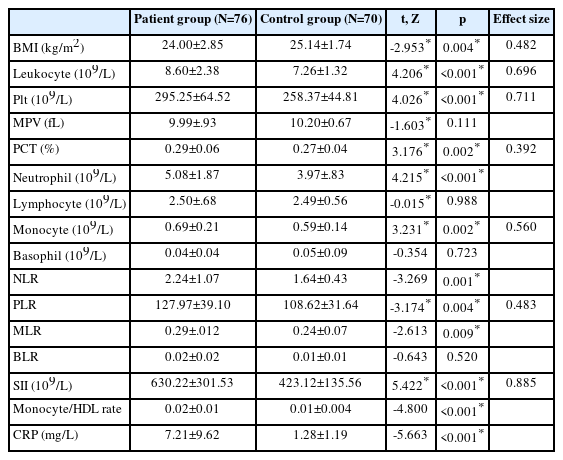
Table 2 compares the BMI and blood parameters of the patient and control groups. The BMI value of the patient group was significantly lower than the control group (p=0.004, effect size; 0.482). Leukocyte (p<0.001, effect size; 0.696), Plt (p<0.001, effect size; 0.711), PCT (p=0.002, effect size; 0.392), neutrophil (p<0.001), monocyte (p=0.002, effect size; 0.560), and CRP (p<0.001) levels in the patient group were significantly higher than in the control group. NLR (p=0.001), PLR (p=0.004, effect size; 0.483), MLR (p=0.009), SII (p<0.001, effect size; 0.885), and monocyte/HDL ratio (p<0.001) were higher in the patient group than in the control group. The effect size of SII was 0.885.
Table 3 shows the relationship between the daily methamphetamine intake, methamphetamine use duration, SII, BMI, and blood parameters. We observed a significant and positive correlation between daily methamphetamine intake and methamphetamine use duration (p=0.002), PCT (p=0.044), neutrophil (p=0.021), NLR (p=0.001), PLR (p=0.004), MLR (p=0.029), and SII (p<0.001).
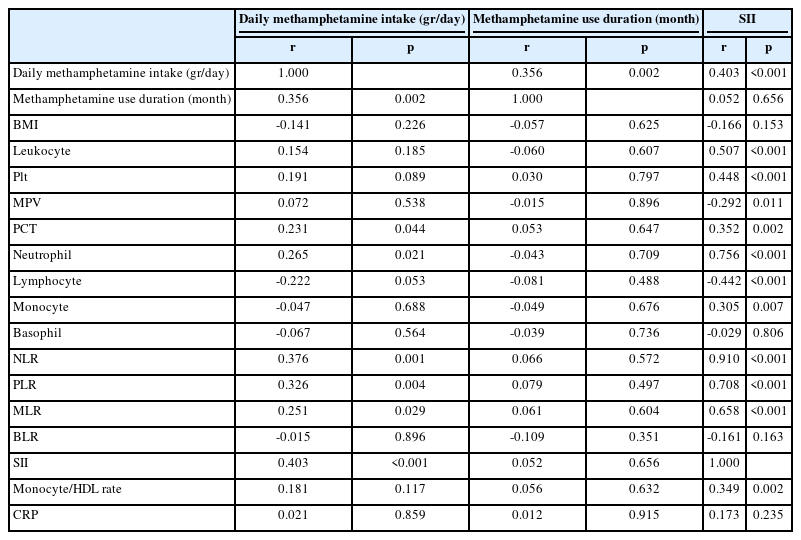
The relationship between daily methamphetamine intake, methamphetamine use duration, SII, BMI, and blood parameters in patients
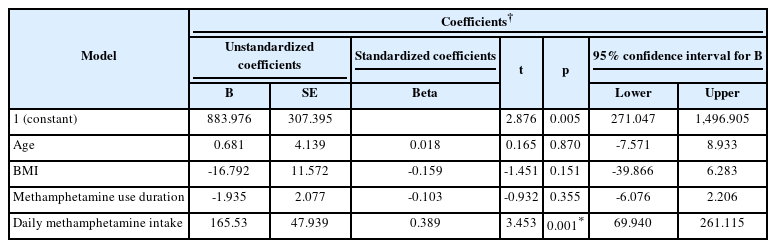
A model of age, BMI, methamphetamine use duration (month), and daily methamphetamine intake (gr/day) was created on SII. According to the linear regression analysis results, only the daily methamphetamine intake was effective on SII (p=0.001). A one-unit increase in daily methamphetamine intake elevated SII by 165.53 units. Linear regression analysis of the effect of age, BMI, methamphetamine use duration and daily methamphetamine amount on SII is shown in Table 4.
DISCUSSION
This study suggests the presence of systemic inflammation in MUD with different markers and variation patterns. It is the first study using routine biochemistry and peripheral hemogram parameters (leukocytes, neutrophils, lymphocytes, monocytes, basophils, MPV, PCT, CRP), monocyte/HDL ratio, NLR, MLR, PLR, BLR, and SII to affirm the subclinical inflammation in adults with MUD through evaluating multiple hematologic parameters.
Methamphetamine addiction is associated with the male gender, a low education level, heavy tobacco use, and increased unemployment [22,23]. Our patient population was male and 53.9% were unemployed. The education level was low, and the majority of the patients were smokers. The behavioral effects of methamphetamine comprise elevated alertness and energy, weight loss, diminished need for sleep, and loss of appetite [24]. In our study, the patient group had a lower BMI than the control group, consistent with the literature.
Although inflammation is crucial to prevent adverse environmental challenges, the inflammatory response can become dysregulated and damage healthy tissues [7,8]. Inflammation is a complex process. Several molecules, including cytokines, acute-phase proteins, and chemokines, directly or indirectly regulate it [7]. Peripheral inflammation may supply neuroinflammation through changes in several organs and tissues. Inflammatory mediators have been linked to numerous psychiatric disorders and contribute to neuroinflammation. Neuroinflammatory processes are often a part of many mental illnesses, such as major depression, bipolar disorder, schizophrenia, or SUD [3,7-9].
Subclinical inflammation and peripheral inflammatory parameters are among the most emphasized areas in recent studies to reveal the etiopathogenesis of psychiatric diseases. Leukocyte (neutrophil, lymphocyte, monocyte, eosinophil, and basophil) levels, MPV, PCT, and CRP values are used to specify an inflammatory response [9,11,12,18,19,25]. NLR, PLR, MLR, SII, and monocyte/HDL ratio are informative parameters that indicate chronic low-grade inflammation [9-11,16,19,25,26]. The literature mentions the role of inflammation in psychiatric diseases such as schizophrenia, mood disorders, obsessive-compulsive disorder, and major depressive disorder [11,26]. In the literature, there are studies on subclinical inflammation in patients with SUD, but the results are inconsistent. NLR was high in patients with cocaine use disorder [10], and PLR and NLR were increased in patients with heroin use disorder [18]. However, NLR and PLR in adults with MUD were low, and this might be related to the possible toxic effects of methamphetamine on the immune system [19]. In our study, the patients’ leukocyte, neutrophil, and monocyte counts; Plt, PCT, and CRP levels; and NLR, PLR, and MLR were higher in the patient group compared to the control group. We observed a significant and positive correlation between the daily methamphetamine intake, methamphetamine use duration, PCT, neutrophil, NLR, and PLR. On the contrary, lymphocyte levels did not vary significantly between the control and patient groups.
MUD is described as causing severe dysregulation of the peripheral immune response [24,27,28]. Pro- and anti-inflammatory cytokine and chemokine expression have been linked to neuronal damage caused by methamphetamine use [29]. Cannabinoid derivatives have anti-inflammatory properties and can modulate immune function [8]. Acute cannabinoid exposure may reduce the cellular immune response, inhibit inflammatory cytokine and chemokine production, and diminish excitotoxicity and neuronal cell damage [30]. Individuals with a history of cannabis use have lower CRP levels (<0.5 mg/dL) compared to current users and never-users [31]. 56,6% of our patients (n=43) had a history of other illicit drug use. Considering that there were cannabis users among them, CRP levels and even other inflammation markers would be much higher.
Macrophages and monocytes are essential in synthesizing and releasing proinflammatory and prooxidant cytokines. HDL performs antithrombotic, anti-inflammatory, and antioxidant activities. It protects the endothelium from the harmful effects of LDL and prevents LDL oxidation [32-34]. The monocyte/HDL ratio may be a novel inflammatory and oxidative stress indicator [16]. SII is a new inflammatory biomarker that accurately reflects the balance between inflammation and immune response [35]. High SII values are associated with disease severity and poor prognosis in many diseases and malignancies [11,14,17]. Patients with major depressive disorder have a high monocyte/HDL ratio which elevates as the severity of depression increases [36]. Recently, the use of SII has been expanded to include psychiatric disorders. SII is elevated mainly in men who express manic episodes and women accompanying depressive episodes [11]. High SII levels have been associated with major depressive disorder [37]. A study on patients with schizophrenia and bipolar affective disorder demonstrated an increased monocyte/HDL ratio and SII [33]. However, we could not find any research on the monocyte/HDL ratio and SII in adults with MUD. We discovered that the monocyte/HDL ratio and SII were higher in the patient group than in the control group. The effect size of SII was 0.885. We found a significant and positive relationship between SII and daily methamphetamine intake. A one-unit increase in daily methamphetamine intake elevated SII by 165.53 units. Our findings reveal the presence of subclinical inflammation in male adults with MUD. The literature reports that methamphetamine disrupts peripheral and central immune functions. Inflammatory cytokines enter the central nervous system and stimulate glial cells to produce similar inflammatory cytokines, which trigger addictive behavior and neurotoxicity [29,38].
Endothelial cell permeability, neutrophils, macrophages, and Plt are all part of the first-line inflammatory response, and their role in inflammation is becoming increasingly important [39]. Plt and PCT levels contribute to vascular events such as atherosclerosis and thrombosis [12,13]. In the current study, the patient group’s Plt and PCT values were significantly elevated compared to the control group. PCT showed a significant positive relationship between daily methamphetamine intake and SII. Methamphetamine increases endothelial surface antigens, which have a crucial role in endothelial damage and are a risk factor for death due to cardiovascular disease [27]. Cardiac complications such as malignant hypertension, arrhythmia, aortic dissection, hemorrhagic stroke, myocardial infarction secondary to vasospasm, and methamphetamine-related cardiomyopathy play a vital role in methamphetamine-related morbidity [24,40,41].
Cytokines, chemokines, and cell adhesion molecules might be involved in methamphetamine-induced neuron damage and cognitive function loss [42]. Ibudilast is a phosphodiesterase and glial activation inhibitor. It alleviates the acute proinflammatory effects of methamphetamine and reduces methamphetamine locomotor activity, addiction, and relapse [30,42-44]. Minocycline, a tetracycline group antibiotic, may lessen the reward effects of methamphetamine and decrease methamphetamine-induced dopamine release, relapse, and neurotoxic effects [30,44]. Ceftriaxone, a β-lactam group antibiotic, reduces cocaine and methamphetamine craving [44]. These studies suggest that drugs that diminish methamphetamine-induced neuroinflammation can also reduce methamphetamine-related neurodegeneration. Thereby, they can enhance neurocognition and treatment results in methamphetamine addiction.
We recommend long-term follow-up of these patients and evaluation of those who develop organic pathology and additional psychopathology to reduce morbidity and mortality. This will help us to understand how immune-inflammatory response enhances the comorbid disease in methamphetamine users and will improve our knowledge of the pathogenesis of these disorders.
The strengths of this study are the strict exclusion criteria, the use of interview-based diagnostic evaluations, the evaluation of the participants by an experienced psychiatrist, the inclusion of important immune system-related variables, the prospective nature of the study, and the compatibility of our findings with the literature. Although addiction is a comorbid disease, excluding patients with major comorbidities is a strength and a limitation of our study. The limitations of our study are that it is cross-sectional, some of the data are based on self-report, only male participants were included, and immune system-related variables, such as cytokines, were not included in our analysis. In addition, smoking may also increase the levels of inflammatory biomarkers. Here, we aimed to reflect a real example of society. It is essential to remember that the study’s results may only represent part of society.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: all authors. Data curation: Nilifer Gürbüzer. Methodology: Nilifer Gürbüzer, Mustafa Can Güler. Formal analysis: Nilifer Gürbüzer, İbrahim Hakkı Tör. Investigation: Nilifer Gürbüzer. Writing—original draft: Nilifer Gürbüzer. Writing—review & editing: Nilifer Gürbüzer, Mustafa Can Güler.
Funding Statement
None
Acknowledgements
The authors would like to thank all the reviewers and the editor for their valuable feedback during the preparation of this manuscript.