Motor Activity in Adult Patients with Attention Deficit Hyperactivity Disorder
Article information
Abstract
Objective
Hyperactivity is a core symptom of attention-deficit hyperactivity disorder (ADHD), but limited information is available on analysis of activity patterns in this disorder. The aim of the study was to analyze motor activity during daily living in adult patients with ADHD.
Methods
Patients (n=76) from the private psychiatric practice of two of the authors were recruited, and were compared to patients with other psychiatric disorders and to normal controls. Actigraphs were used to record motor activity for six days, with one minute intervals, and data were analysed using linear and non-linear mathematical methods.
Results
For short recording periods (300 minutes) the activity levels of ADHD patients do not differ from normal controls, but the autocorrelation (lag 1) is lower and Fourier analysis shows higher power in the high frequency range, corresponding to the period from 2-8 min. During recordings for six days there are no significant differences between ADHD patients and the control groups. The combined and inattentive subgroups differ only in the six days recordings. The Fourier analyses show that the combined type has lower power in the high frequency range, corresponding to the period from 4-8 hours, and in the analysis of rhythms the intra-daily variability is lower, compared to the inattentive type.
Conclusion
Adult ADHD patients do not show evidence of hyperactivity, but have levels of activity similar to normal controls. However, on several measures ADHD patients display altered activity patterns, indicating that the regulation of motor activity in this disorder is different from controls.
INTRODUCTION
Attention-deficit hyperactivity disorder (ADHD) is defined by symptoms of hyperactivity, impulsivity and inattention but in addition other symptoms are also prevalent in patients with this disorder, such as mood instability, frustration intolerance and sleep problems.123 In psychiatric practice these patients are mainly seen in an out-patient setting, and co-morbidity with mood and anxiety disorders is the rule rather than the exception.4 The core symptom hyperactivity has a basis in behavioural observations,1 but increased activity levels have also been documented with objective registrations using actigraphs56 and infrared motion analyses.7 It is generally assumed that hyperactivity decreases with age,14 but a recent study in adults described increased motor activity as a more discriminative feature of the disorder than either inattention or impulsivity.7 On the other hand, the notion that there is a pervasive hyperactivity in children with ADHD has been questioned.8
There is limited information regarding a more detailed analysis of motor activity patterns in ADHD. Teicher5 reported that actigraphically recorded hyperactivity was caused by the absence of quiet periods rather than to periods with increased activity. Mathematical methods such as Fourier analysis, to describe the frequency spectrum, and sample entropy, to measure of the complexity of time series, have been used to characterize the movement pattern of patients with depression and schizophrenia.910 The aim of the present was to use these methods to study the motor activity of adult ADHD patients. We wanted to analyze data from extended time periods (6 days) and record activity during daily living, since a majority of previous investigations have been performed in a laboratory setting for short (hours) time periods,6 and it is conceivable that other patterns may emerge when studying motor activity in a more natural setting.
METHODS
Ethics statement
The study protocol was approved by the Norwegian Regional Medical Research Ethics Committee West. Written informed consent was obtained from all participants involved in the study.
Subjects
Patients were recruited from the private psychiatric practice of KM and WF, both certified psychiatrists with long clinical experience and on contract with the Western Norway Regional Health Authority. The patients were consecutive new referrals, in need of diagnostic evaluation of either ADHD or mood/anxiety disorders, and age between 18 and 65 years.
Exclusion criteria were inability to speak Norwegian and not being able to comply with the study protocol. A total of 104 patients were recruited. For different reasons we were not able to obtain recordings for 27 patients, partly due to logistics problems and partly to patients forgetting to wear the actigraphs. We therefore had 77 actigraph recordings performed. One of the patients had been treated with stimulants during testing and was therefore omitted from the analyses, bringing the number of patients to 76, and these are reported on in the present paper. The group consisted of 35 women and 41 men, and the average age was 37.6±10.9 years (mean±SD), range 17-61.
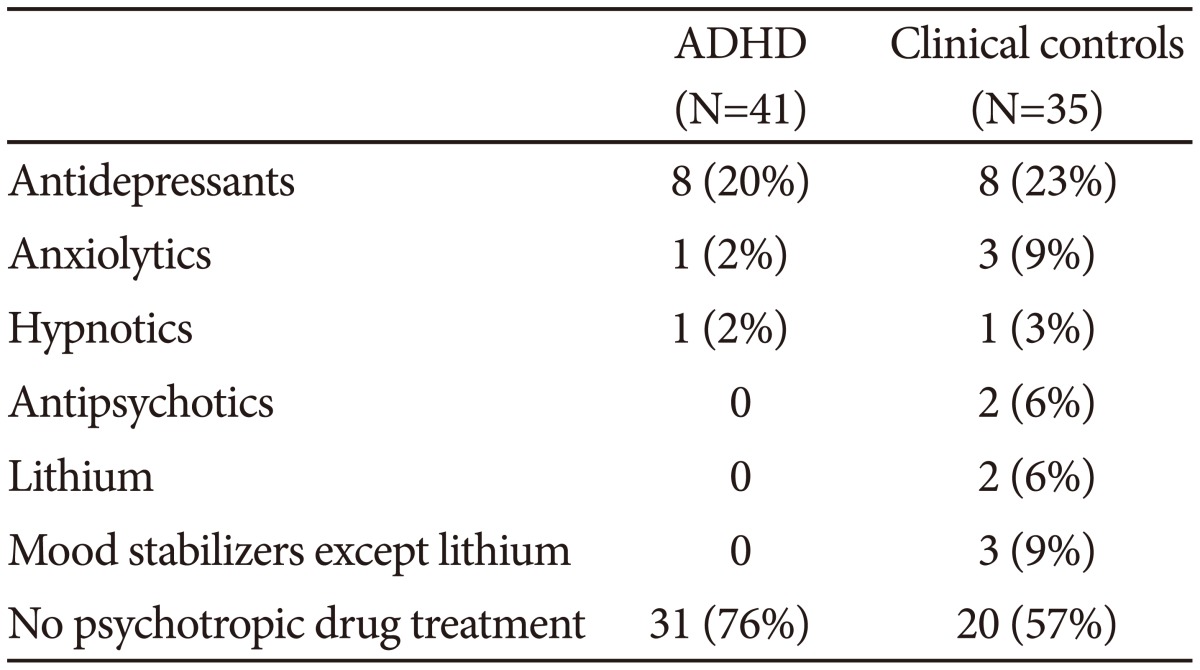
Most of the patients used no psychotropic drugs, 76% of the ADHD patients and 57% of the clinical controls. Patients using drugs at referral continued unchangesd with these during the actigraph recordings. A summary of the drugs used is presented in Table 1.
The control group consisted of 20 women and 12 men, average age 38.4±11.2 years, range 21-66, medical students (n=5), patients without serious medical or psychiatric symptoms from a primary care office (n=4) and employees from a psychiatric nursing home (n=23). None of the control subjects had a history of affective or psychotic symptoms. The controls were recruited during a separate study, using the same actigraph equipment as the patients in this study, and are reported on in two previous papers.911
Psychiatric assessment
All diagnostic assessments of the patients were performed by either KM or WF using a standard clinical interview, supplemented when necessary and possible with information from collateral sources. In addition the following assessment instruments were employed:
The Mini-International Neuropsychiatric Interview (MINI Plus, version 5.0.0), a module based semi-structured interview for DSM-IV and ICD-10 diagnoses.12,13
The Montgomery-Asberg Depression Rating Scale (MADRS), a standard instrument for the assessment of depression.14
Adult ADHD Self-Report Scale (ASRS). This is the World Health Organization's rating scale designed to measure current symptoms of ADHD in adults. It consists of 18 questions that follow the DSM-IV criteria for ADHD, and uses a 5 point scale of 0-4 (0=never, 4=very often), with a possible range of scores from 0-72. Items 1-9 cover symptoms of inattention and 10-18 hyperactivity and impulsivity.151617
Wender Utah Rating Scale, 25 questions version (WURS-25), a 25-item self-rating scale designed to assess symptoms and signs of ADHD in childhood, using a Likert scale of 0-4 (0=never, 4=very often), yielding a possible score range of 0-100.18 WURS-25 has been used in previous studies in Norway.17
Mood Disorder Questionnaire (MDQ). This is a screening instrument for bipolar disorder that has been validated both in clinical and control populations. It is a self-report form that consists of 13 questions scored "Yes" of "No". Positive answer to at least 7 questions and confirmation that the symptoms have occurred together and caused problems is suggestive of a bipolar disorder.1719
Cyclothymic temperament scale, a self-report form that consists of 21 questions covering the cyclothymic temperament according to the definition of Akiskal.2021 This scale is part of the larger TEMPS-A autoquestionnaire.22
Hospital Anxiety and Depression Scale (HADS). This is a self-assessment form for detecting current states of depression and anxiety, and has been extensively used, also in Norway.23
Conner's Continuous Performance Test II, a test frequently used in the evaluation of ADHD.24
The final diagnostic evaluation assessment was made after an assessment of all available information, and a consensus diagnosis, based on DSM-IV criteria, was made after discussion of each case (KM, WF, OBF, and JØB).
Recording of motor activity
Motor activity was monitored with an actigraph worn at the right wrist (Actiwatch, Cambridge Neurotechnology Ltd, England). In the actigraph, activity is measured by using a piezoelectric accelerometer that is programmed to record the integration of intensity, amount and duration of movement in all directions. The sampling frequency is 32 Hz and movements over 0.05 g will be recorded. A corresponding voltage is produced and is stored as an activity count in the memory unit of the actigraph. The number of counts is proportional to the intensity of the movement. The right wrist was chosen to make the procedure more convenient for the participants, since most of them have their watches around the left wrist and it is cumbersome to have two such devices on the same arm. Previous studies have shown that there are small differences between the right and left wrist.2526 Total activity counts were recorded for one minute intervals for a period of minimum six days. For each of these actigraph recordings raw data was inspected, and if they contained periods longer than one hour without any activity during daytime (07:00-23:00), the recordings were not used for analyses employing the whole period of six days.27
Mathematical analyses
For all the time periods we calculated seven measures: The mean activity, the standard deviation (SD), expressed as percent of the mean; the root mean square successive differences (RMSSD), expressed as percent of the mean; the relation between RMSSD and SD (RMSSD/SD); the autocorrelation (lag 1); sample entropy and Fourier analysis. The software used for the estimation of sample entropy was from the Physio Toolkit Research Resource for Complex Physiologic signals,28 see http://www.physionet.org.
In recordings both from patients and controls there are shorter and more prolonged periods of inactivity. To obtain time series with continuous motor activity we searched manually each recording and selected a period with a length of 300 min, containing not more than 4 consecutive minutes with zero activity, by searching from the start of the recording and using the first period that satisfied this criterion. In this way we were able to obtain 300 min sequences from each participant. In addition data were analysed using information from the whole recording period (six days), with activity counts for one half hour as the unit of measurement. For these time periods we also calculated three additional measures, related to analyses of rhythms.
Sample entropy
For the analysis of sample entropy the data were normalized, by transforming the time series to have sample mean 0 and sample variance 1. Sample entropy is a nonlinear measure, indicating the degree of regularity (complexity) of time series, and is the negative natural logarithm of an estimate of the conditional probability that subseries of a certain length that match point-wise, within a tolerance, also match at the next point. We chose the following values, m=2 and r=0.2. Sample entropy was employed since it can be employed with comparatively short time series (>50) and is robust with regard to outliers.29
Fourier analysis
Data were normalized before analysis. No windows were applied. For analysis of the data from the 300 min periods, the first 256 min were used. Results are presented as variance (% of the total variance) in four components of the spectrum: 6.5×10-5-0.0010 Hz (corresponding to the period from 16-256 min); 0.0010-0.0021 Hz (8-16 min), 0,0021-0,0042 Hz (4-8 min), and 0.0042-0.0083 Hz (2-4 min). For analysis of data for 6 days, the first 256 half hour periods were used (128 hrs). Results are presented as variance (% of the total variance) in four components of the spectrum: 1.93×10-6-3.47×10-5 Hz (corresponding to the period from 8-128 hrs), 3.47×10-5-6.94×10-5 Hz (4-8 hrs), 6.94×10-5-1.39×10-4 Hz (2-4 hrs), and 1.39×10-4-2.78×10-4 Hz (1-2 hrs).
Analyses of rhythms
Three nonparametric variables developed for analysis of actigraph data were used;
Inter-daily stability (IS), intra-daily variability (IV), and relative amplitude (RA).27 The IS quantifies the invariability between the days, that is, the strength of coupling of the rhythm to supposedly stable environmental factors. The IV gives an indication of the fragmentation of the rhythm, that is, the frequency and extent of transitions between rest and activity. RA is calculated using data from the most active 10 h period and the least active 5 h period in the average 24 h pattern. For the analyses of IS, IV and RA data from the whole period of six days were used.
Statistics
ANOVA and Student's t-test were employed to evaluate differences between groups, with the p-value set at 0.05. For the ANOVA analyses post hoc Bonferroni tests were used to compare ADHD or clinical controls with the normal controls. SPSS version 18 was used for the statistical analyses.
RESULTS
After a comprehensive evaluation and consensus discussion, 41 patients received a diagnosis of ADHD, and 35 were deemed not to fulfill the criteria for ADHD, and all were given other psychiatric diagnoses (clinical controls). The gender ratio (female/male) is, 17/24 in the ADHD group and 18/17 in the clinical control group. The mean age is similar in the two groups (37.2±10.8 vs. 38.0±11.2 years).
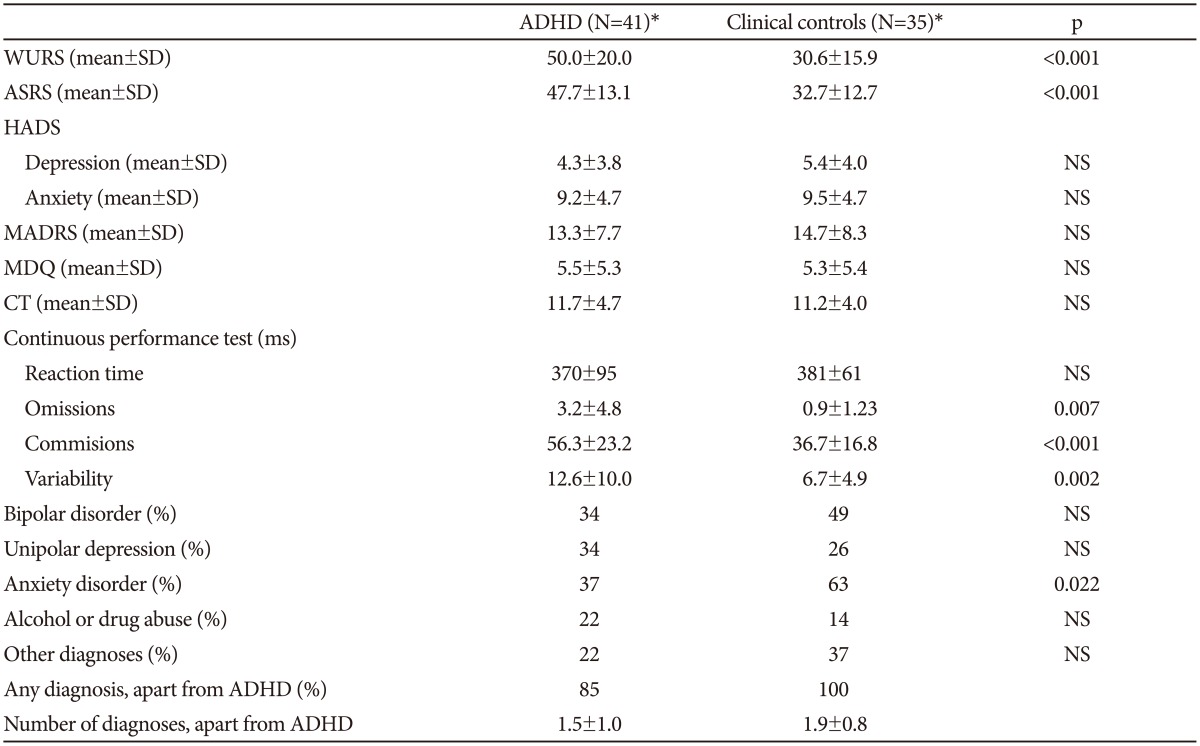
In Table 2 the clinical characteristics of the two groups are shown. The ADHD group has substantially higher scores on both the WURS and ASRS scales than the controls, and, apart from the reaction time, results from the continuous performance test are also clearly different in the two groups. For the questionnaires and the MADRS scale there are no significant differences between the groups. All but seven of the ADHD patients also had another psychiatric diagnosis. There are a higher number of patients with anxiety disorders in the group without ADHD, but for the other diagnosis groups there are no significant differences between the groups.
In the ADHD group 18 patients fulfilled criteria for the combined subtype and 23 for the inattentive type. The patients with the combined type have higher scores on the WURS scale, but apart from that there are no significant differences between the groups (Table 3).
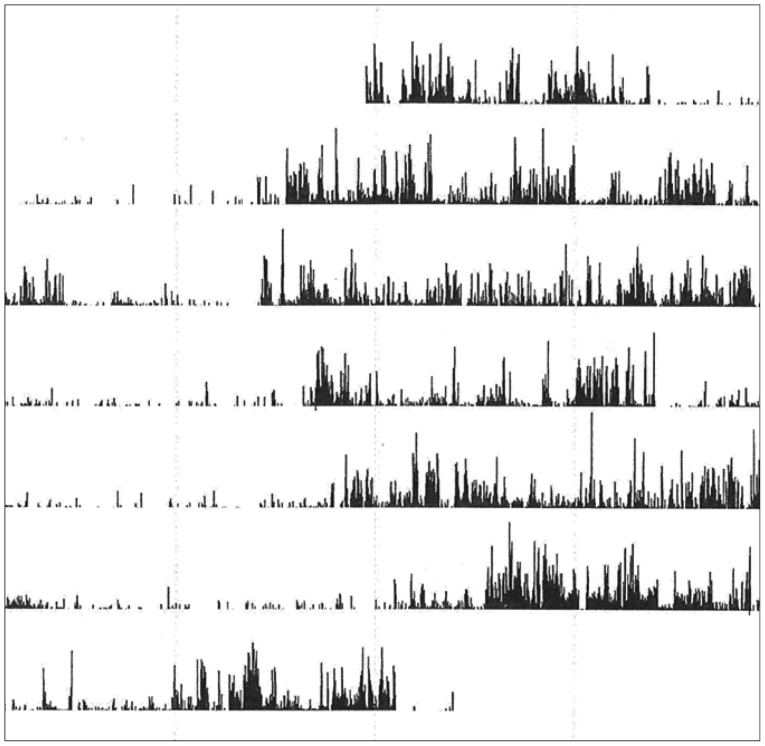
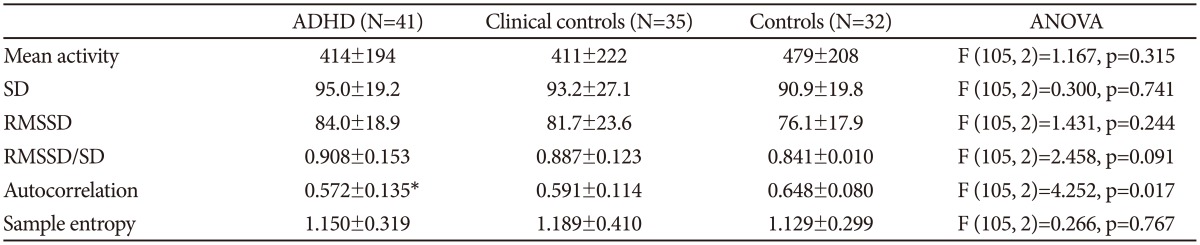
In Figure 1 are shown an actigraph recording from an ADHD patient (during seven days), and in Figure 2A the activity profile from a 300 min sequence with continuous motor activity. In Table 4 the results from actigraphic recordings of 300 min periods with continuous motor activity are presented. The mean activity level, SD, RMSSD, the RMSSD/SD ratio, and the sample entropy, are not significantly different across the three groups. However, the autocorrelation is lower in the ADHD group, compared to the normal controls.
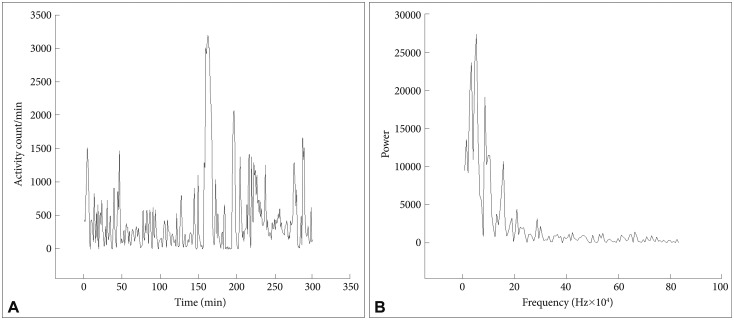
A and B: Activity counts during 300 min from an ADHD patient. Fourier analysis of a 256 min recording (from the actigraph recording in A). ADHD: attention-deficit hyperactivity disorder.
Fourier analysis of these 300 min sequences (Figure 2B) also revealed clear differences between the groups, with the ADHD group showing higher power in the high frequency range, corresponding to the periods of 2-4 and 4-8 min (Table 5).
When analyzing actigraphic recordings for six days we were able to use data from 32 patients with ADHD, 29 clinical controls and 20 normal controls. There were no significant differences between the three groups for any of the measures analyzed (data not shown).
Comparing the two ADHD subgroups reveals no significant differences with regard to analyses of the 300 min recordings. Concerning the six days recordings the mean activity, SD, RMSSD, RMSSD/SD, autocorrelation and sample entropy are not significantly different (data not shown), but in the Fourier analysis the combined type have lower power in the high frequency range, and this is significant for the 6.94×10-5-1.39×10-4 Hz frequency range, corresponding to the time period 2-4 hours, with higher power in the 1.93× 10-6-3.47×10-5 Hz frequency range, corresponding to the period from 8-128 hrs. In addition the rhythm analyses show that the combined type also has a significantly lower intradaily variability compared to the inattentive type (Table 6).
DISCUSSION
Using objective registration of motor activity during daily living we have not been able to find any evidence of pervasive hyperactivity in adult patients with ADHD, either in continuous periods with motor activity or in total activity counts during six days. However, on several measures ADHD patients display altered activity patterns, indicating that the regulation of motor activity is indeed altered in this disorder. For periods with continuous motor activity, using a recording period of one minute, the ADHD patients are characterized by increased variability in the high frequency part of the spectrum, corresponding to the periods from 2-8 min in the Fourier analysis, and at the same time the autocorrelation (lag 1) is reduced. The RMSSD/SD ratio is increased compared to normal controls, but not significantly so. The clinical controls have values on these measures between the normal controls and the ADHD patients. We have seen a similar pattern of motor variability changes assessed with actigraphs in patients with schizophrenia;910 and in healthy controls given the NMDA-antagonist memantine,10 indicating that altered motor activity in patients with schizophrenia may be related to changes in NMDA receptor function. An example of timedependent changes in variability pattern in ADHD is the findings of Castellanos et al.30 that oscillations in the reaction time of ADHD patients were different from controls, with a frequency of 0.05 Hz (cycle length 20 sec). They attributed this to altered catecholaminergic neurotransmission. Most of the neurochemical changes found in ADHD are related to dopaminergic systems,31 but with regard to the close interaction between glutamate and dopamine in the brain32 and the possibility that glutaminergic systems are affected in ADHD33 we may speculate that similar changes in NMDA receptor function are related to the motor disturbances in ADHD. In schizophrenic patients, in addition to the changes in variability, sample entropy was increased compared both to controls and to depressed patients,9 while administration of memantine did not influence sample entropy values.10 In the present study we found no change in this measure of complexity, neither in the ADHD patients nor in the clinical controls. We are not aware of other studies that have used this method (or others) to measure the degree of order or complexity in time series of motor activity.
When analyzing the whole period of six days, using sequences of 30 minutes, we did not find any significant differences between ADHD patients and the two control groups. These data therefore show that changes in variability differ, depending on the time frame used to analyze data. A pattern of reduced total activity and increased SD was found in depressed patients in our previous study,9 and increased variability of motor activity has been described by Wood et al.34 in children with the combined type of ADHD. Increased intra-individual variability has also been found in different neuropsychological tests in patients with ADHD,35 depression and schizophrenia,36 and in registration of mood in depressed patients.37 Alterations in variability patterns are therefore not limited to one diagnostic category or one test method, but increased intra-individual variability has been suggested to be related to cognitive decline in the elderly and to degenerative brain disorders,38 and possibly connected with alterations in dopaminergic systems.39
Pervasive hyperactivity is a defining characteristic of ADHD,4 and increased motor activity has been documented in children with ADHD using objective measures, such as actigraphs, mostly during short,634404142 but also with more prolonged recording periods.54344 Similarly, hyperactivity of children with ADHD has been documented in laboratory settings using infrared motion analysis.45 Increased motor activity has also been found in adult patients with ADHD, mostly during short recordings, connected with neuropsychological testing,74647 but also in a week-long study.48 In addition, treatment both with methylphenidate and atomoxetine has been shown to reduce hyperactivity.464950 However, the notion that there is a continuous hyperactivity in ADHD patients has been challenged by Licht and Tryon who found that only one of nine children diagnosed with the combined form of ADHD was pervasively hyperactive.8 The present results are compatible with those findings. Furthermore, Teicher5 notes that increased activity of children with ADHD are due more to the absence of quiescent periods than to periods with high activity.
The lack of difference between the combined and the inattentive types of ADHD for most of the activity measures, including total activity counts, is in agreement with the finding of Dane et al.42 that activity scores in children, measured with actigraphs, did not distinguish between these subtypes. Similarly, Teicher7 using infrared motion analysis in adult ADHD patients did not find any difference in degree of hyperactivity between the two types. However, there are two measures where the two ADHD subtypes differ in our study. For the whole period of six days, using sequences of 30 minutes, Fourier analysis shows that, compared to the inattentive type, the combined type has significantly lower power in the frequency range corresponding to the period of 4-8 hours, and at the same time the intradaily variability, using van Someren's rhythm analysis27 is lower. The intra-daily variability is calculated using one hour time intervals and is therefore in broad agreement with the Fourier analysis results. Together these findings indicate differences in organization of motor activity between the two subtypes, with the combined type having a less variable pattern, in the time range of a few hours. Boonstra et al.,48 using the same analysis method, also found that adult ADHD patients, all with the combined or hyperactive form of ADHD, had reduced intra-daily variability. However, in addition they found increased inter-daily stability, both of these findings indicating a more stable and less variable motor activity pattern. This pattern of reduced intra-daily variability and increased inter-daily stability is similar to what we found in the sample of schizophrenic patients described above.11
Actigraph recording is of course giving only a crude measure of activity level, compared to a more detailed analysis using infrared motion analysis. However, as documented above, hyperactivity has previously been found using both types of equipment, so we do not think that our inability to find evidence of hyperactivity is due to our choice of actigraphs for measurement. Additionally, to be able to study activity level over more prolonged time periods and in daily living actigraphs are more convenient.
The patients recruited for the present study are not necessarily representative of ADHD patients in the general population. Most of them have other psychiatric disorders and it is therefore difficult to disentangle the contribution of ADHD from the effect of these other conditions. On the other hand, comorbidity is the rule rather than the exceptions when meeting these patients in an adult psychiatric setting, and also in epidemiological studies the rate of comorbidity is high.4 We therefore think that the study group may be representative for adult ADHD patients presenting for evaluation in a psychiatric out-patient clinic.
Even though the clinical interviews were performed by two different psychiatrists, the use of the same diagnostic instruments and a final evaluation of each case by four experienced psychiatrists make it likely that we have obtained a reasonable accurate diagnostic assessment for the whole study sample.
Conclusion
Using actigraphic assessment of motor activity in adult ADHD patients do not show any evidence of hyperactivity, but these patients have levels of activity similar to normal controls. However, using one minute recording periods, we find a pattern of variability similar to previous findings in schizophrenic patients and in healthy controls given the NMDA-antagonist memantine, indicating that motor activity in ADHD is altered, possibly related to changes in glutamatergic neurotransmission. The combined subtype differ from the inattentive type in having a less variable activity pattern in the time range of hours, again similar to what we have seen in schizophrenic patients. These results underscore the importance of further studies related to the function of motor systems in this common neurodevelopmental disorder.
Acknowledgments
This research has been supported by the Norwegian Resource Center for ADHD, Tourette Syndrome and Narcolepsy.