Validation of the Dementia Care Assessment Packet-Instrumental Activities of Daily Living
Article information
Abstract
Objective
We aimed to evaluate the psychometric properties of the IADL measure included in the Dementia Care Assessment Packet (DCAP-IADL) in dementia patients.
Methods
The study involved 112 dementia patients and 546 controls. The DCAP-IADL was scored in two ways: observed score (OS) and predicted score (PS). The reliability of the DCAP-IADL was evaluated by testing its internal consistency, inter-rater reliability and test-retest reliability. Discriminant validity was evaluated by comparing the mean OS and PS between dementia patients and controls by ANCOVA. Pearson or Spearman correlation analysis was performed with other instruments to assess concurrent validity. Receiver operating characteristics curve analysis was performed to examine diagnostic accuracy.
Results
Chronbach's α coefficients of the DCAP-IADL were above 0.7. The values in dementia patients were much higher (OS=0.917, PS=0.927), indicating excellent degrees of internal consistency. Inter-rater reliabilities and test-retest reliabilities were statistically significant (p<0.05). PS exhibited higher reliabilities than OS. The mean OS and PS of dementia patients were significantly higher than those of the non-demented group after controlling for age, sex and education level. The DCAP-IADL was significantly correlated with other IADL instruments and MMSE-KC (p<0.001). Areas under the curves of the DCAP-IADL were above 0.9.
Conclusion
The DCAP-IADL is a reliable and valid instrument for evaluating instrumental ability of daily living for the elderly, and may also be useful for screening dementia. Moreover, administering PS may enable the DCAP-IADL to overcome the differences in gender, culture and life style that hinders accurate evaluation of the elderly in previous IADL instruments.
INTRODUCTION
The decline of functional activities is the critical feature in the diagnosis of dementia.1 Functional assessment instruments measure activities of daily living (ADL), a measure of a person's ability to live independently. Impaired ADL can be disclosed by clinical interviews. Patients or their families complain about forgetting appointments, difficulties in managing money or dressing themselves, inability to use telephone and so on. ADL assessment is also an important outcome measure in evaluating efficacy of anti-dementia drugs. Along with cognitive measures, ADL measures are commonly included in clinical trials of anti-dementia drugs as a primary outcome measure.2-4
ADL can be divided into basic ADL (BADL) and instrumental ADL (IADL). BADL include self-maintenance skills such as bathing, dressing and toileting. IADL refers to daily activities that are not necessary for fundamental functioning, but enable the individual to live independently within a community (light housework, preparing meals, taking medications, shopping, using the telephone, managing money and so on).5 IADL, in particular, requires greater complexity of neuropsychological organization. IADL is more likely to be vulnerable to cognitive decline,5-9 and thus its impairment may be an important indicator of early dementia.2,3,10-12 Some studies showed that IADL measures are useful screening instruments for dementia.13-15 In addition, IADL can also reflect the level of dependency and prognosis of dementia patients.2-4
IADL can be measured in three ways: self-reported questionnaires, performance-based assessment and informantbased questionnaires.16 Self-reported questionnaires are difficult to accomplish for dementia patients since most dementia patients can not aware their functional impairment.17-20 Observation or direct assessment has the advantage of directly obtaining information without relying on self- or informantreport. Nonetheless, the major drawback of this method is the time-consuming aspect of these instruments.2,21 Hence, the most common method for assessing dementia is the informant-based questionnaires. Currently, various informantbased questionnaires for IADL are available and their number is still growing.2,22 Also, a number of Korean instruments are available for IADL.23-25
Despite the widespread use of these assessment tools, there are some limitations in the current measures of IADL. First, some measures include items that are relatively unrelated to IADL (memory, comprehension, or social functioning).24,25 Second, some include items that are not easily applicable to men (for example, grooming).23-25 Third, some include both IADL and BADL items together.16 Finally, most scales do not score the items that the subject have not experienced within an observation window. This scoring system may make pose difficulties in comparing IADL directly between and within subjects, and result in underestimation of potential functional ability if subjects pursuit a simple lifestyle.
To overcome these limitations in the currently available IADL measures, the IADL measure included in the Dementia Care Assessment Packet (DCAP-IADL)26 included only IADL items that are equally applicable to both genders and asked to score not only the items that subjects experienced but also the items that subjects have not experienced within an observation window. In the present study, we evaluated the psychometric properties and usefulness of the DCAP-IADL in evaluating dementia patients.
METHODS
Subjects
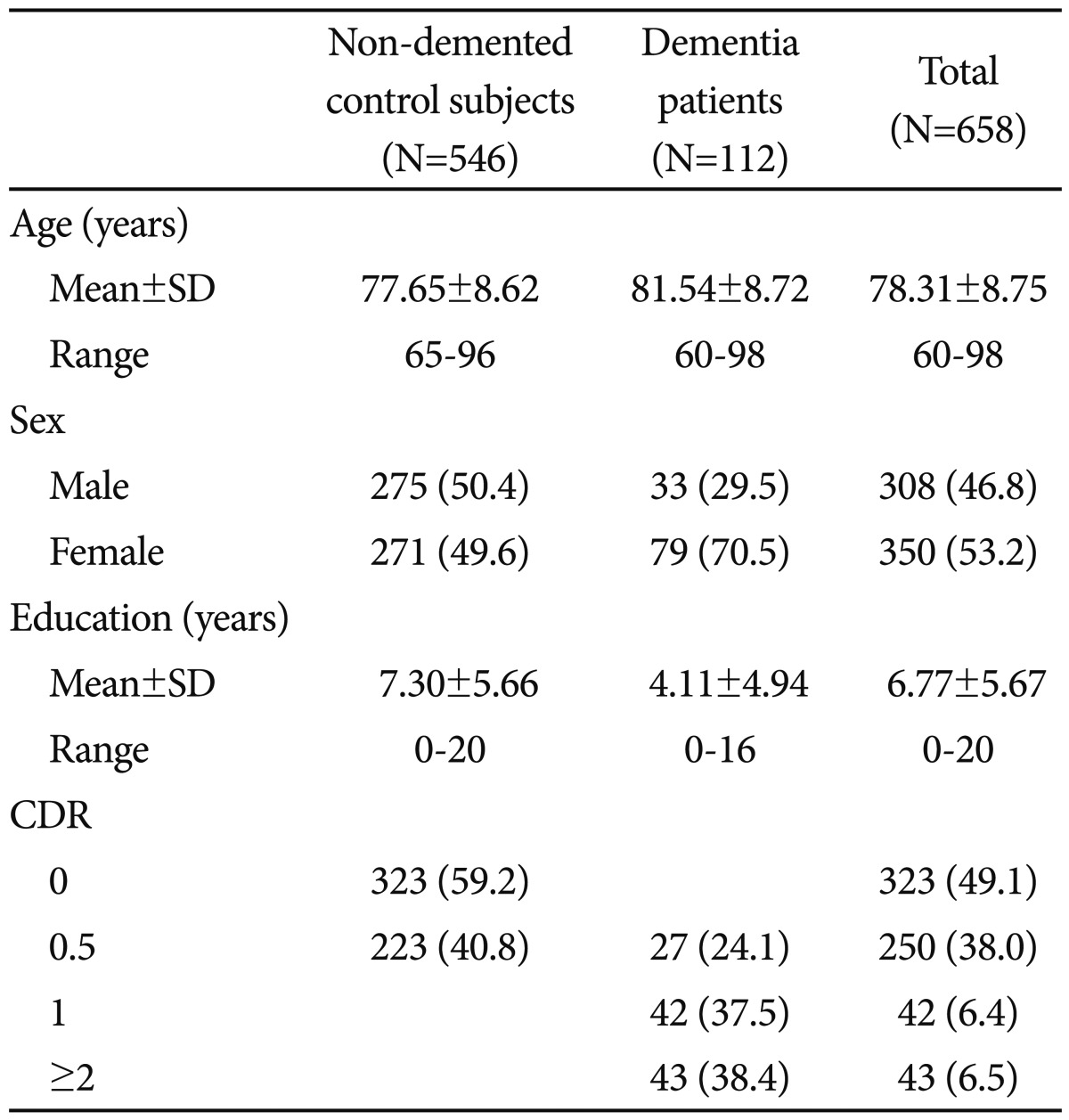
The participants consisted of 112 dementia patients (79 Alzheimer's disease, 25 vascular dementia, 1 frontotemporal dementia, 4 dementia of Lewy body disease, 4 Parkinson's disease dementia, and 3 other dementia) and 546 non-demented elderly controls aged 60 years or older. The non-demented elderly subjects were community-dwelling Korean elders who participated in the Korean Longitudinal Study for Health and Aging (KLoSHA).27 The dementia patients were recruited from either the visitors to the Dementia Special Clinic of Dankook University Hospital (n=58) or the participants of the KLoSHA (n=54). All subjects were evaluated according to the clinical assessment protocol of the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment battery (CERAD-K).28 The diagnosis of dementia was made according to the DSM-IV diagnostic criteria.29 All dementia patients had reliable informants who could provide adequate clinical histories. Among the dementia patients, 27 (24.1%), 42 (37.5%) and 43 (38.4%) were very mild (CDR=0.5), mild (CDR=1) and moderate to severe (CDR=2 or 3), respectively.
The demographic characteristics of the subjects are presented in Table 1. The dementia patients were older (p<0.001) and less educated (p<0.001) than the non-demented controls. Women were more common in the dementia group than in the non-demented control group (p<0.001).
DCAP-IADL
The DCAP-IADL consists of a total of 10 items that include 3 community items (mode of transportation, going outside within walking distance, and shopping), 5 personal management items (ability to use the telephone, ability to use electric appliances, ability to handle money, ability to manage personal belongings, and being responsible for own medication), and, 2 household items (housekeeping and food preparation). "Laundry", although it is an important and common activity of daily living, is not included since it is barely performed by men in Asian cultures. All items are rated based on observations in the recent 90 days. Each item is rated from 0 to 3 points. The total score of the DCAP-IADL is 30 points, and a higher score indicates lower instrumental activities of daily living. We scored the DCAP-IADL in two ways: observed score (OS) and predicted score (PS). For scoring OS, informants were asked to score each item based on observations in the recent 90 days. If an item could not be observed during this time period, it was scored as 3 points, which is equivalent to "cannot be carried out". However, in scoring PS, informants were asked to give their best guesses on the items that they have not experienced instead of giving 3 points. For instance, if a female subject did not manage financial matters because her husband takes exclusive charge of handling finance, we requested her informant to predict whether she would be able to manage financial matters if her husband did not take on the job.
Reliability
Three aspects of reliability were examined internal consistency of scale, inter-rater reliability and test-retest reliability. Internal consistency was examined by Crohnbach's alpha. In order to evaluate inter-rater reliability, 36 dementia patients and 8 non-dementia controls were rated with the DCAP-IADL simultaneously by two independent researchers (tester and rater). The DCAP-IADL was administered again to 17 dementia patients and 13 non-dementia controls by the same rater at 2-4 weeks after the initial assessment. For evaluating inter-rater and test-retest reliabilities, Pearson correlation coefficients were calculated.
Validity
To evaluate discriminant validity, the mean OS and PS were compared between the dementia patients and non-demented subjects by ANCOVA after adjusting for age, sex and educational level. We evaluated the concurrent validity of the DCAP-IADL by calculating its Pearson correlation coefficients with K-IADL,30 BDS-ADL,28 Korean version of Mini Mental Status Examination (MMSE-KC),28 and the sum of the box scores of the Clinical Dementia Rating (CDR-SOB)31 and a Spearman correlation coefficient with CDR.
To examine diagnostic accuracy, we performed receiver operating characteristics (ROC) curve analysis for both OS and PS of the DCAP-IADL and compared their areas under the ROC curves (AUC) with those of K-IADL and MMSE-KC.32
All statistical analyses were performed using SPSS for windows, version 13.0 and Medcalc for windows, version 12.2.1.0 (MedCalc Software, Mariakerke, Belgium).
RESULTS
Reliability
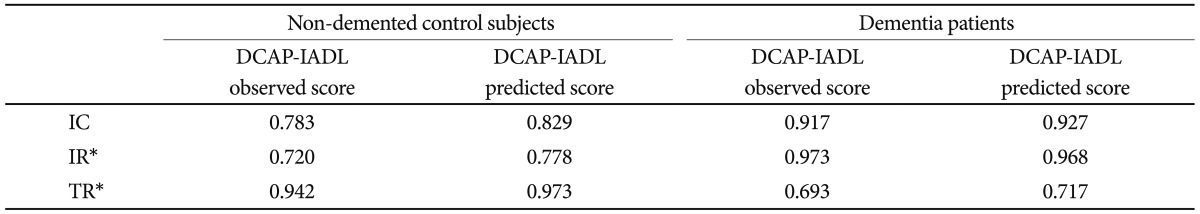
The results of the reliability analysis are presented in Table 2. Chronbach's coefficients alpha for OS and PS in non-demented control subjects were both above 0.7 (OS=0.783, PS=0.829). The values in dementia patients were much higher (OS=0.917, PS=0.927), indicating excellent degrees of internal consistency. 33 Inter-rater reliabilities for OS and PS in the non-demented control subjects, as measured by Pearson correlation coefficients, were both statistically significant (OS=0.720, PS=0.778, p<0.05). The values in dementia patients were much better (OS=0.973, PS=0.968, p<0.05). Test-retest reliabilities for OS and PS in the non-demented control subjects were both statistically significant (OS=0.942, PS=0.973, p<0.05). Those in dementia patients were both statistically significant (OS=0.693, PS=0.717, p<0.05), however lower than those in the non-demented controls. Internal consistency and test-retest reliabilities for PS of the DCAP-IADL in the non-dementia subjects and the dementia patients were higher than those for OS. Inter-rater reliabilities for PS of the DCAP-IADL in the non-dementia subjects were higher than those for OS, and inter-rater reliabilities for PS of the DCAP-IADL in the dementia patients were comparable to those for OS. As a whole, PS of the DCAP-IADL exhibited higher reliabilities than OS.
Validity
As shown in Table 3, the DCAP-IADL clearly discriminated the dementia group from the non-demented group. The mean OS and PS of dementia patients were significantly higher than those of the non-demented group after controlling for age, sex and education level (p<0.0001, ANCOVA). The mean scores of OS (df=4, F=266.55, p<0.0001) and PS (df=4, F=314.74, p<0.0001) of the DCAP-IADL for the 3 different dementia stages according to CDR indices were significantly different (Figure 1). In post-hoc multiple comparison analyses, all of the different dementia stages differed significantly from one another (p<0.005, Bonferroni comparison). As summarized in Table 4, OS and PS of the DCAP-IADL were significantly correlated not only with other functional measures such as K-IADL (OS=0.840, PS=0.845, p<0.001), BDS-ADL (OS=0.784, PS=0.815, p<0.001), and CDR-SOB (OS=0.791, PS=0.816, p<0.001) but also with global cognitive measures such as MMSE-KC (OS=-0.704, PS=-0.718, p<0.001). OS and PS of the DCAP-IADL were also correlated with global severity measures such as CDR (OS=0.612, PS=0.631, p< 0.001). The AUC of OS and PS for dementia were 0.931 (95% CI=0.900-0.963) and 0.940 (95% CI=0.912-0.969), respectively (Table 5). The optimal cut-off point for dementia was 7/8 (sensitivity=0.902, specificity=0.837) in OS and 6/7 (sensitivity=0.902, specificity=0.841) in PS, indicating that both OS and PS may have excellent diagnostic accuracy for dementia. 34 The AUC of OS and PS for dementia were comparable to that of the K-IADL (AUC=0.921, 95% CI=0.893-0.949) and MMSE-KC (AUC=0.919, 95% CI=0.892-0.946) (Figure 2).
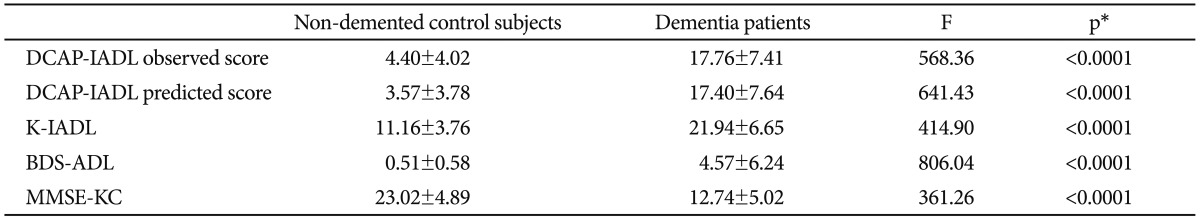
Mean scores of DCAP-IADL, K-IALD, BDS-ADL, and MMSE-KC in non-demented control subjects and dementia patients
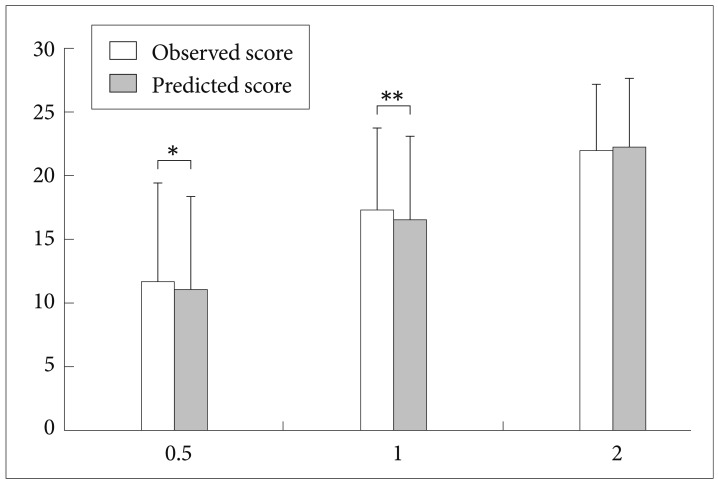
Mean scores on the Dementia Care Assessment Packet Instrumental Activities of Daily Living (DCAP-IADL) for the 3 different dementia stages according to CDR severity indices. All mean scores of the observed score and predicted score of DCAP-IADL differed significantly from one another by post hoc analysis (Bonferroni comparison, p<0.05). *p=0.013 by paired t-test, **p=0.001 by paired t-test. CDR: Clinical Dementia Rating scale.
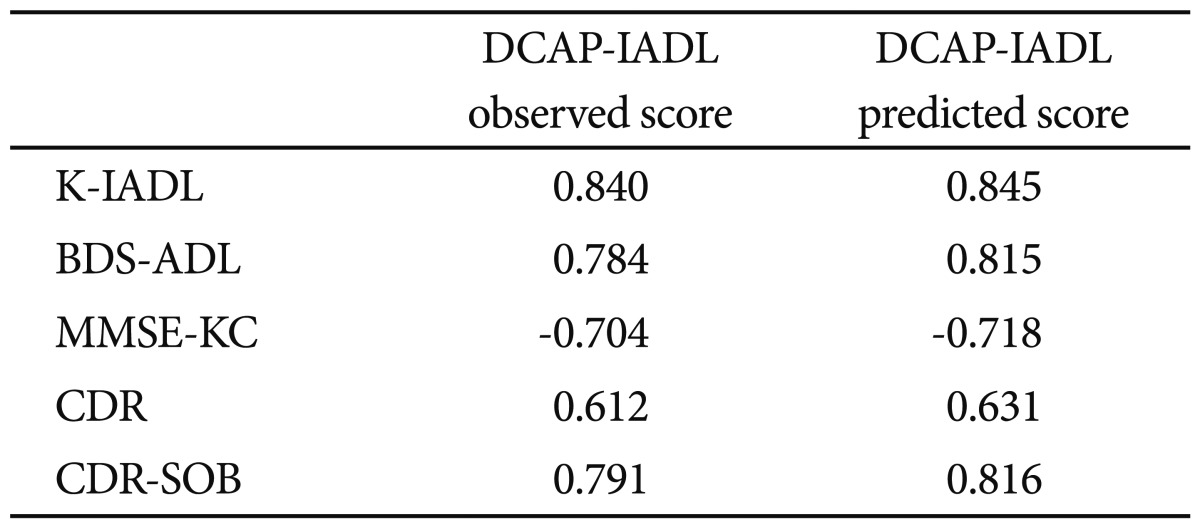
Correlation between the Dementia Care Assessment Packet Instrumental Activities of Daily Living (DCAP-IADL) and other instruments for evaluating activities of daily living, global cognition and global severity of dementia
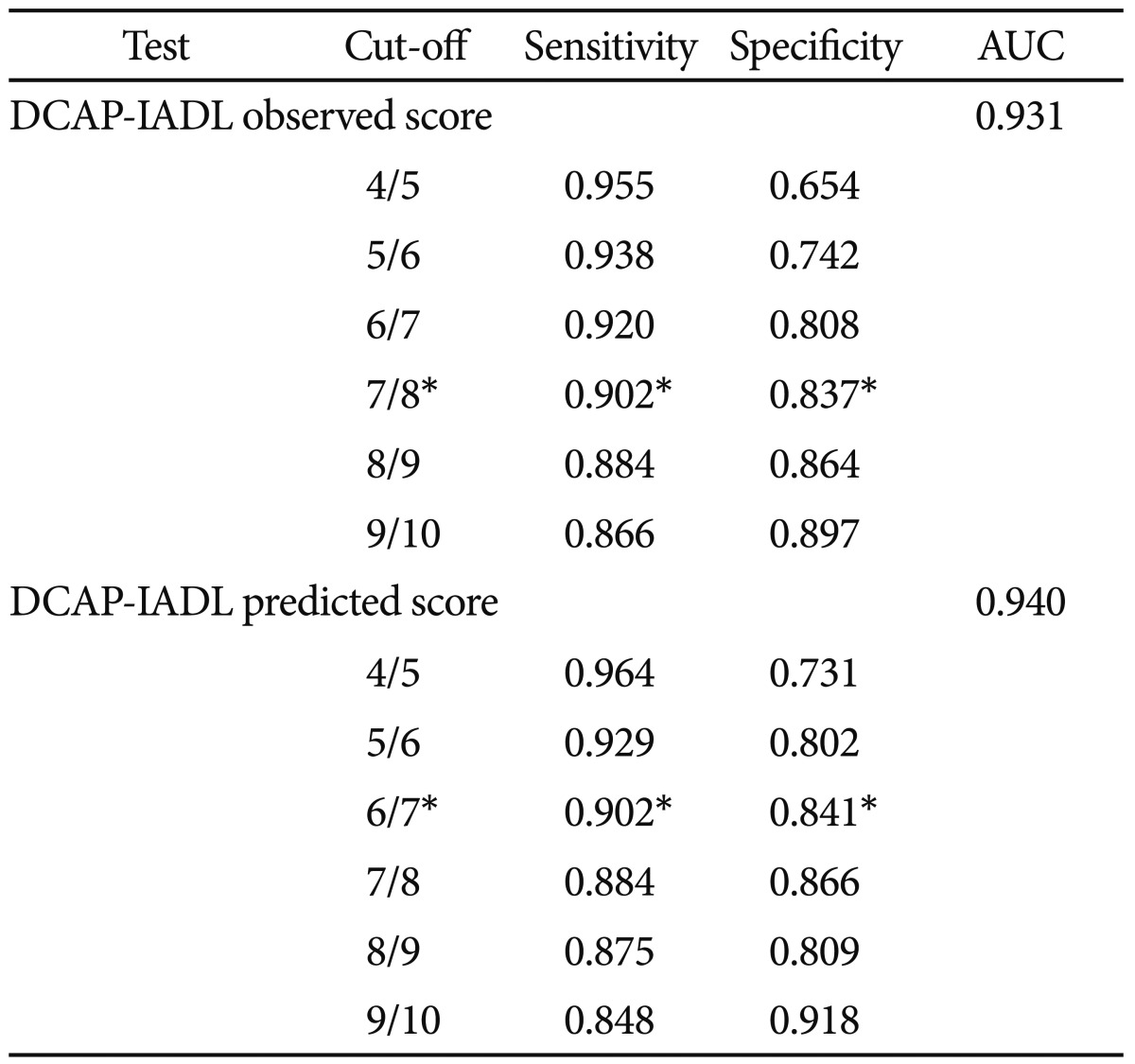
Sensitivity and specificity of the Dementia Care Assessment Packet Instrumental Activities of Daily Living (DCAP-IADL) for diagnosis of dementia patients
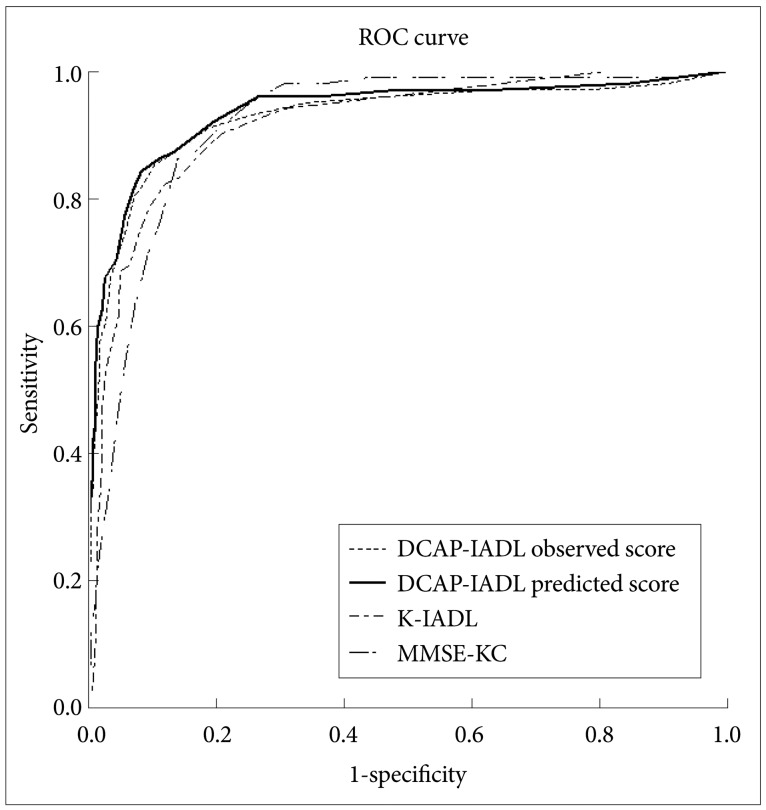
The area under curve (AUC) of DCAP-IADL observed score, DCAP-IADL predicted score, K-IADL, and MMSE-KC. DCAP-IADL: the Dementia Care Assessment Packet Instrumental Activities of Daily Living, K-IADL: Korean Instrumental Activities of Daily Living, MMSE-KC: the Korean MMSE version of the Consortium to Establish a Registry for Alzheimer's disease, ROC: Receiver Operating Characteristics.
DISCUSSION
In the present study, the DCAP-IADL was found to be a reliable and valid instrument for evaluating functional abilities of the elderly. A noteworthy result was that the reliability and validity of PS were comparable to or slightly better than those of OS. Most informant-based IADL instruments include some items that are not equally applicable to both genders in some cultures or not applicable in certain life styles. For example, in general, men do not prepare meals and women do not handle finances in many Asian cultures. Elders who lived with their children or dwell in nursing homes may not perform housekeeping duties, laundry, and food preparation by themselves. These items that subjects do not normally experience were not rated or given a minimum score in most previous IADL instruments. However, the DCAP-IADL excluded or minimized items that could be influenced by gender, culture, dwelling environment and life style. Moreover, to evaluate items that subjects have not experienced within an observation window, we used PS simultaneously with OS instead of excluding that item from the overall score or giving it a minimum score. In the PS of the DCAP-IADL, we asked informants to make their best guesses for the IADL items that could not be directly observed. Pre-existing tools did not adopt the scoring system like PS due to the concern of informant bias. However, our results suggest that the discriminant and concurrent validity of PS were comparable to or slightly better than those of OS. As Figure 1 shows, in comparison between OS and PS for the 3 different dementia stages according to CDR indices, OS was shown to be significantly higher than PS in very mild (p=0.013, paired t-test) and mild dementia (p=0.001, paired t-test). On the other hand, in more than moderate dementia, no difference was observed between OS and PS (p=0.208, paired t-test). These results suggest that OS might rate impairment of IADL in dementia patients much more severely than PS in early stage dementia. The reason for this discrepancy between OS and PS might be that the measurement of IADL was more influenced by the characteristics of subjects in early stage dementia. Therefore, PS may better reflect realistic IADL of dementia patients than OS especially those in early stage dementia. By minimizing the items that might be influenced by the characteristics of subjects (gender, culture, lifestyle and so on) and rating the items that were not actually performed in their everyday lives although they could performed such tasks in required situations, it is expected that inter-individual and intra-individual comparisons are easier and consistent in the evaluation of IADL. Except Lawton and Broody scale,5 the DCAP-IADL is a tool containing questionnaires only for IADL. Most previous tools such as the Alzheimer's Disease Cooperative Study-Activities of Daily Living scale (ADCS-ADL), 35 BDS-ADL,28 the Bristol Activities of Daily Living scale (Bristol ADL),36,37 the Cleveland Scale for Activities of Daily Living (CSADL),38 the Disability Assessment for Dementia scale (DAD)39,40 and Interview for Deterioration of Daily living in Dementia (IDDD)41,42 include items probing both ADL and IADL. Even Alzheimer's Disease Cooperative Study-Activities of Daily Living-Prevention Instrument (ADCS-ADL-PI)43 include items for checking memory (ability to remember important dates and times, such as appointments or meetings), vision, hearing and mobility (ability to walk two blocks or further without help). Inclusion of items not related to IADL might cause unnecessary overlap of evaluation and difficulties in interpreting the results. To evaluate IADL solely, the DCAP-IADL excluded the items that were relatively unrelated to IADL (memory, comprehension and social functioning) and related to BADL. Nevertheless, our results showed that the DCAP-IADL (both OS and PS) have excellent diagnostic accuracy for dementia and is able to efficiently discriminate 3 different dementia stages. Barberger-Gateau et al. suggested that four IADL items (telephone use, use of transportation, responsibility for own medication, and handling money) are correlated with cognitive impairment independent of age, sex, and education.13 Furthermore, one year follow-up showed that the odds ratio for incident dementia, adjusted for age, increased from 10.6 for subjects dependent on one IADL to 318.4 for those dependent on four IADLs, compared with independent subjects.14 The baseline score of four IADLs was also a predictor of 3-year but not of 5-year incident dementia.15 For the purpose of screening dementia, global cognition tests, such as MMSE, are universally used. Although the DCAP-IADL was not originally designed as a screening instrument for dementia, the AUC of OS and PS for dementia are 0.931 (95% CI=0.900-0.963) and 0.940 (95% CI=0.912-0.969), respectively. These are comparable to and slightly higher than the AUC of MMSE-KC (AUC=0.919, 95% CI=0.892-0.946), in spite of no significant difference. This suggests that the DCAP-IADL may be a useful tool for screening dementia. Moreover, evaluation of IADL by the DCAP-IADL is relatively simple and less time-consuming which makes this a clinically more feasible tool. IADL is important not only for evaluating dementia, but also for evaluating mild cognitive impairment (MCI). MCI is considered to be a prodromal stage of Alzheimer's disease (AD) and other forms of dementia.44-46 Petersen et al.47 proposed the following diagnostic criteria for MCI: 1) Not normal, not demented; 2) self and/or informant report and impairment on objective cognitive tasks; 3) Evidence of decline over time on objective cognitive tasks; and 4) Preserved basic activities of daily living/Minimal impairment in complex instrumental functions. However, these criteria have not been applied uniformly across many studies47,48 due to variations in utilizing the diagnostic criteria.49,50 "Minimal impairment in complex instrumental functions" among the diagnostic criteria was also an ambiguous criteria because there was no definite guideline for minimal impairment in IADL. In the presented study, we suggest an optimal cut-off point of 7/8 (sensitivity 90%, specificity 84%) for OS of the DCAP-IADL and an optimal cut-off point of 6/7 (sensitivity 90%, specificity 84%) for PS of the DCAP-IADL. We can use these cut-off points as guidelines for minimal impairment in IADL. That is, over 7 (OS) or over 6 (PS) can be defined as impaired IADL in the dementia level and 1 to 6 (OS) or 1 to 5 (PS) can be defined as minimally impaired IADL in the MCI level. However, the limitations of the present study warrant further studies on the DCAP-IADL. First, the demographic characteristics of the subjects were not equal between the non-demented controls and dementia patients. As mentioned earlier in the report, the dementia patients were older and less educated than the non-demented controls. Women were more common in the dementia group than in the nondemented control group. These differences might influence on our results even though influence might be minimum level. Second, various dementia patients (Alzheimer's disease, vascular dementia, Dementia of Lewy body disease, Parkinson's disease dementia and so on) were enrolled in the present study. Diversity of dementia subjects might also influence on our results because physical disability in early stage vascular dementia or Parkinson's disease dementia, might have contributed to the DCAP-IADL scores rather than resulting in cognitive decline. Third, the present study was cross-sectional investigation. Thus, longitudinal studies on advanced dementia patients are needed to determine whether the DCAP-IADL can validly detect cognitive decline over time.
In conclusion, the DCAP-IADL is a reliable and valid instrument for evaluating the instrumental ability of daily living for the elderly, and may also be useful for screening dementia. Moreover, administering PS may enable the DCAP-IADL to overcome the differences in gender, culture and life style that hinder accurate evaluation in previous IADL instruments.
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry for Health, Welfare, & Family Affairs, Republic of Korea (Grant No. A092077) and by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST; Grant No. 2011-0018262). Also, this study was supported by an independent research grant from AstraZeneca Pharmaceuticals (Grant No. RC200811-399). The sponsors had no role in shaping the design, conduct, interpretation or summary of this research.