Blonanserin Augmentation of Atypical Antipsychotics in Patients with Schizophrenia-Who Benefits from Blonanserin Augmentation?: An Open-Label, Prospective, Multicenter Study
Article information
Abstract
Objective
The purpose of this study was to investigate the efficacy and tolerability of atypical antipsychotics (AAPs) with augmentation by blonanserin in schizophrenic patients.
Methods
aA total of 100 patients with schizophrenia who were partially or completely unresponsive to treatment with an AAP were recruited in this 12-week, open-label, non-comparative, multicenter study. Blonanserin was added to their existing AAP regimen, which was maintained during the study period. Efficacy was primarily evaluated using the Positive and Negative Syndrome Scale (PANSS) at baseline and at weeks 2, 4, 8, and 12. Predictors for PANSS response (≥20% reduction) were investigated.
Results
The PANSS total score was significantly decreased at 12 weeks of blonanserin augmentation (-21.0±18.1, F=105.849, p<0.001). Moreover, 51.0% of participants experienced a response at week 12. Premature discontinuation of blonanserin occurred in 17 patients (17.0%); 4 of these patients dropped out due to adverse events. The patients who benefited the most from blonanserin were those with severe symptoms despite a treatment with a higher dose of AAP.
Conclusion
Blonanserin augmentation could be an effective strategy for patients with schizophrenia who were partially or completely unresponsive to treatment with an AAP.
INTRODUCTION
Schizophrenia is a chronic and disabling mental disorder characterized by severe behavioral symptoms that commonly require life-long therapeutic intervention. While antipsychotic medications are the cornerstone of schizophrenia treatment, the effect of treatment is limited by unfavorable side effects, nonresponse to medication, and modest efficacy on negative symptoms.1 Up to 70% of patients do not achieve full remission, even when taking antipsychotic medications as recommended.2 This limited therapeutic effect of antipsychotic medications continues to be a significant clinical and public health problem.34
In order to overcome the limited effectiveness of antipsychotic drugs, polypharmacy is very common in the treatment of schizophrenia. In fact, it has been reported that 4.1–48.0% of patients use two or more medications, with most studies report a prevalence of between 10% and 30%.56 In a recent study, over 20% of schizophrenic patients in Korea had been prescribed antipsychotic polypharmacy.5 Polypharmacy is popular despite the consensus statements that recommend monotherapy as the standard treatment for schizophrenia,78910 and the fact that there is little clinical data to support it.11 Moreover, there are concerns about the long-term safety,1213 mortality,1415 and increased cost associated with antipsychotics polypharmacy.16
However, antipsychotic polypharmacy could be effective for patients with schizophrenia when a single agent does not adequately relieve symptoms. Pharmacologically, antipsychotic combinations seek to achieve greater therapeutic potential by optimizing dopamine D2 receptor occupancy or increasing activity across a wider range of receptors related to the pathogenesis of schizophrenia.1718 In a review by Chan and Sweeting19 on the combination of non-clozapine second generation antipsychotics with possibly complementary receptor profiles, they reported that some symptom improvement had occurred. Also, beneficial effects of antipsychotic polypharmacy was reported in small, open-label trials with olanzapine plus risperidone20 and olanzapine plus amisulpride.21 Moreover, in a meta-analysis, Correll et al.6 presented findings arguing that antipsychotic polypharmacy may have a clinical advantage over standard (non-clozapine) monotherapy in nonresponsive patients. They also noted that more studies combining non-clozapine atypical antipsychotics (AAPs) with each other, which is a strategy often used in clinical practice, are required to explore the merits of combining antipsychotics in the acute phase instead of waiting at least 10 weeks to declare that nonresponse has occurred.6
Blonanserin is a well-known second-generation antipsychotic commonly used in Japan and Korea with a unique pharmacological receptor profile; it has a higher dopamine D2 receptor occupancy (Ki=0.142 nM) and lower serotonin 2A receptor-blocking activity (Ki=0.812 nM) than other second generation antipsychotics.22 It also has weak dopamine D1 and weak adrenergic alpha1 receptor blocking activity.23 The effectiveness of blonanserin monotherapy in the treatment of schizophrenia has been demonstrated by several clinical studies24252627 and a meta-analysis.28 In a study with Korean schizophrenic patients,25 blonanserin showed comparable effectiveness with risperidone, was more tolerable, and had a better safety profile, particularly with respect to prolactin elevation. Moreover, blonanserin showed the lowest discontinuation rate due to intolerance than other AAPs including quetiapine, aripiprazole, risperidone, perospirone, and olanzapine.29 These results suggest that blonanserin may have advantages in tolerability, one of the most disadvantageous factors for antipsychotic polypharmacy.30
The goals of this study were to test the hypothesis that blonanserin augmentation would improve psychotic symptoms and be well tolerated in patients who failed to respond to another AAP and to identify factors that can be used to predict a patients' response to blonanserin augmentation therapy.
METHODS
Study design and sample
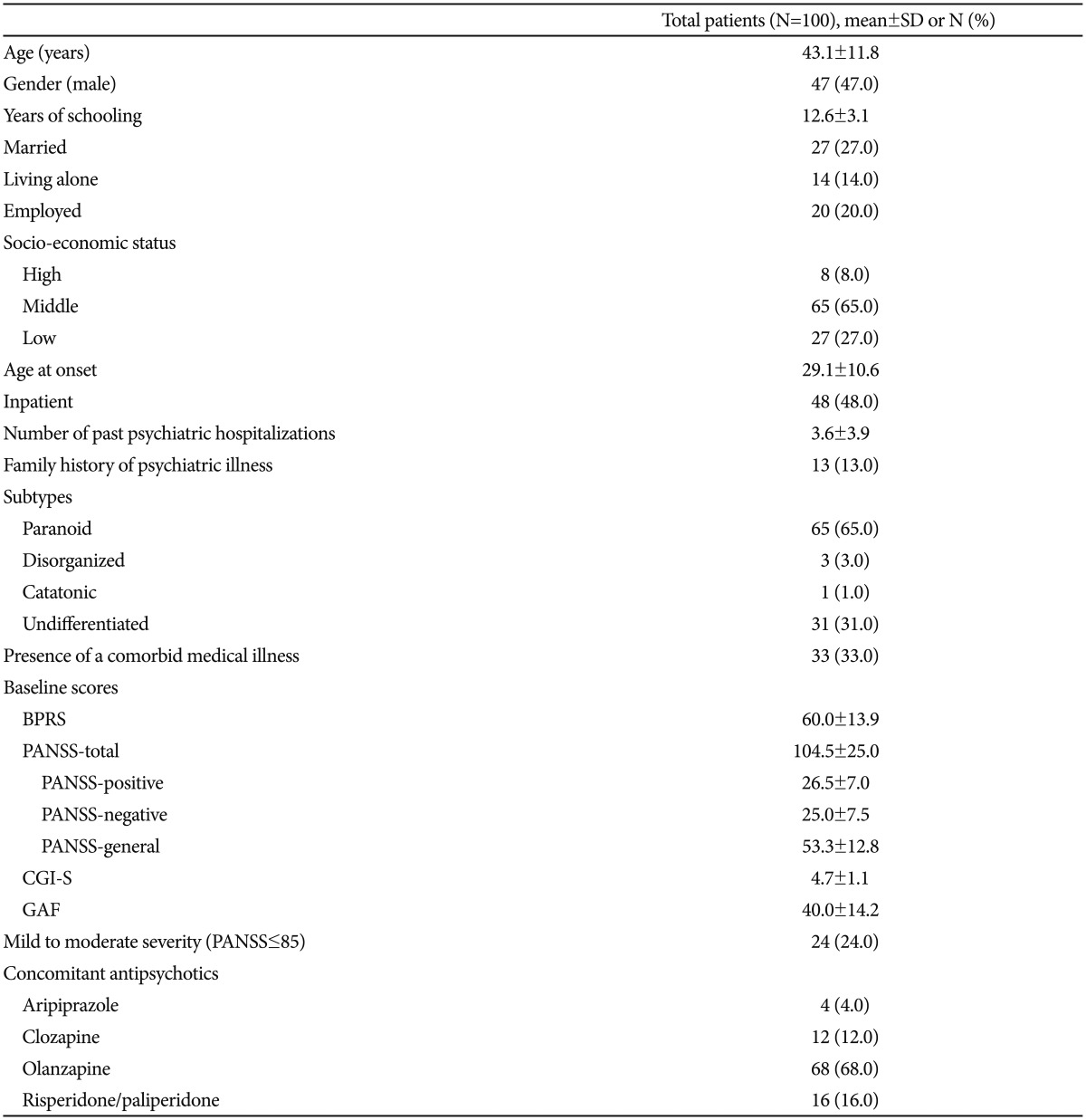
This was an open-label, prospective, multicenter, 12-week study that included 100 patients. The patients were diagnosed with schizophrenia according to DSM-IV-TR criteria and did not respond or only partially responded to an AAP treatment. This study was conducted at 7 centers in Korea, including 5 university hospitals and two psychiatric hospitals.
After at least 6 weeks of treatment with recommended dose of AAPs, patients completed the 18-item Korean version of the brief psychiatric rating scale (BPRS, items scored on a 1–7 scale). Patients scoring 43 or more were classified as non-responders or partial responders.31 At the study entry, participants were ≥20 and ≤70 years of age and were being treated with one AAP: aripiprazole, clozapine, olanzapine, paliperidone, or risperidone. These AAPs were selected because of their pharmacokinetic properties. Blonanserin is known to be metabolized predominantly via the cytochrome P450 (CYP) enzyme CYP3A4 and does not inhibit any key CYP enzymes.32 Therefore, blonanserin is considered unlikely to have any significant clinical pharmacokinetic interactions with antipsychotics that are mainly metabolized by CYP1A2 (clozapine, olanzapine) or CYP2D6 (aripiprazole, risperidone);33 paliperidone does not undergo significant hepatic metabolism.3234 Among the exclusion criteria were pregnancy or lactation, a medical condition that could interfere with everyday life activities, and a diagnosis of any Axis I disorder other than schizophrenia. Patients were excluded from participation if they had been prescribed blonanserin before the study, had been prescribed a depot of antipsychotics within 30 days of the study; were currently being treated with two or more antipsychotics; or were known to be treatment resistant. All patients gave informed consent before participation in the study.
Medication
The blonanserin was recommended to be dosed flexibly within the range of 8–24 mg/day. During the study period, it was recommended that the AAP dose to be kept constant. Concomitant treatment with ongoing mood stabilizers/anticonvulsants or antidepressants was permitted if the patient had been on a stable dose for at least 2 weeks prior to enrollment, but it was requested that the dose not be changed during the study period. Patients were not given any other antipsychotics, mood stabilizers/anticonvulsants, or antidepressants during the study. Benzodiazepines, anti-Parkinsonian agents, and hypnotics were permitted at the discretion of the investigator.
Efficacy measures
Psychiatric symptoms were evaluated with the Korean version of BPRS, the Positive and Negative Syndrome Scale (PANSS), and the Clinical Global Impressions-Severity Scale (CGI-S) at baseline and at weeks 2, 4, 8, and 12. The primary efficacy assessment was defined as mean change from baseline to week 12 on the PANSS total score. Additional efficacy measures included response rates with PANSS and BPRS, mean change in PANSS-positive, negative, and general subscale, BPRS, and CGI-S scores. Response was defined as a decrease in the PANSS and BPRS total scores ≥20%.28
Safety and tolerability
Safety assessments included monitoring of vital signs and weight at baseline and week 12, a physical examination, and reported adverse events at each visit. At each visit, extrapyramidal symptoms were assessed using the Simpson-Angus Scale (SAS) and the Barnes Akathisia Rating Scale (BARS). The Abnormal Involuntary Movements Scale (AIMS) was applied to assess abnormal involuntary movement.
Statistical analysis
Data were analyzed on an intent-to-treat (ITT) group, and the last-observation-carried forward (LOCF) method was applied for endpoint analysis. The data included all patients who provided a baseline and at least one post-baseline data measurement. All subjects who received at least one dose of the study medication were included in the safety analysis.
Categorical variables are presented as absolute number and relative frequencies (%), and nominal variables are described as mean±standard deviation. Data for each psychometric scale and scale for adverse event scores were analyzed by repeated measure analysis of variance (RM-ANOVA). A Greenhouse-Geisser correction was used to test for non-sphericity. The chi-square test or Fisher's exact test was used to analyze categorical variables, and a paired t-test was performed for variables measured at baseline and endpoint.
We also investigated the predictors of response to blonanserin augmentation by comparing responders and non-responders with an independent t-test, chi-square test, or Fisher's exact test. A binary logistic regression analysis was conducted with age, sex, and variables with significance (p<0.05) or trend toward significance (p<0.10) on univariate analyses with response as an independent variable (i.e., responders and non-responders). All statistical tests were two-tailed with a significance level of 0.05.
Ethics
The study was conducted according to the Declaration of Helsinki and Good Clinical Practices. Written informed consent was obtained from all subjects after the subjects were given an extensive explanation of the nature and procedures of the study. The study protocol was approved by the Institutional Review or Ethics Committees at each study site.
RESULTS
Patients and medications
The number of participants who completed the 12-week treatment was 83 (83.0%). The demographic and clinical characteristics of the subjects at baseline are presented in Table 1. The mean chlorpromazine equivalent dose35 of concomitant AAPs at baseline was 516.4±153.6 mg/day. The blonanserin dose at each visit is shown in Figure 1. The mean dose of blonanserin during the study period was 11.7±5.8 mg/day. There were 19 (19.0%) subjects who were treated with concomitant mood stabilizer/anticonvulsants and 8 (8.0%) who were treated with concomitant antidepressants at baseline.
Efficacy and tolerability in the entire sample
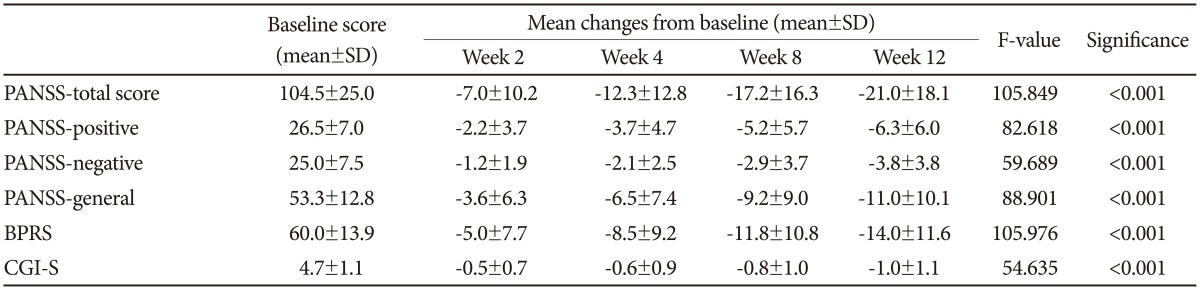
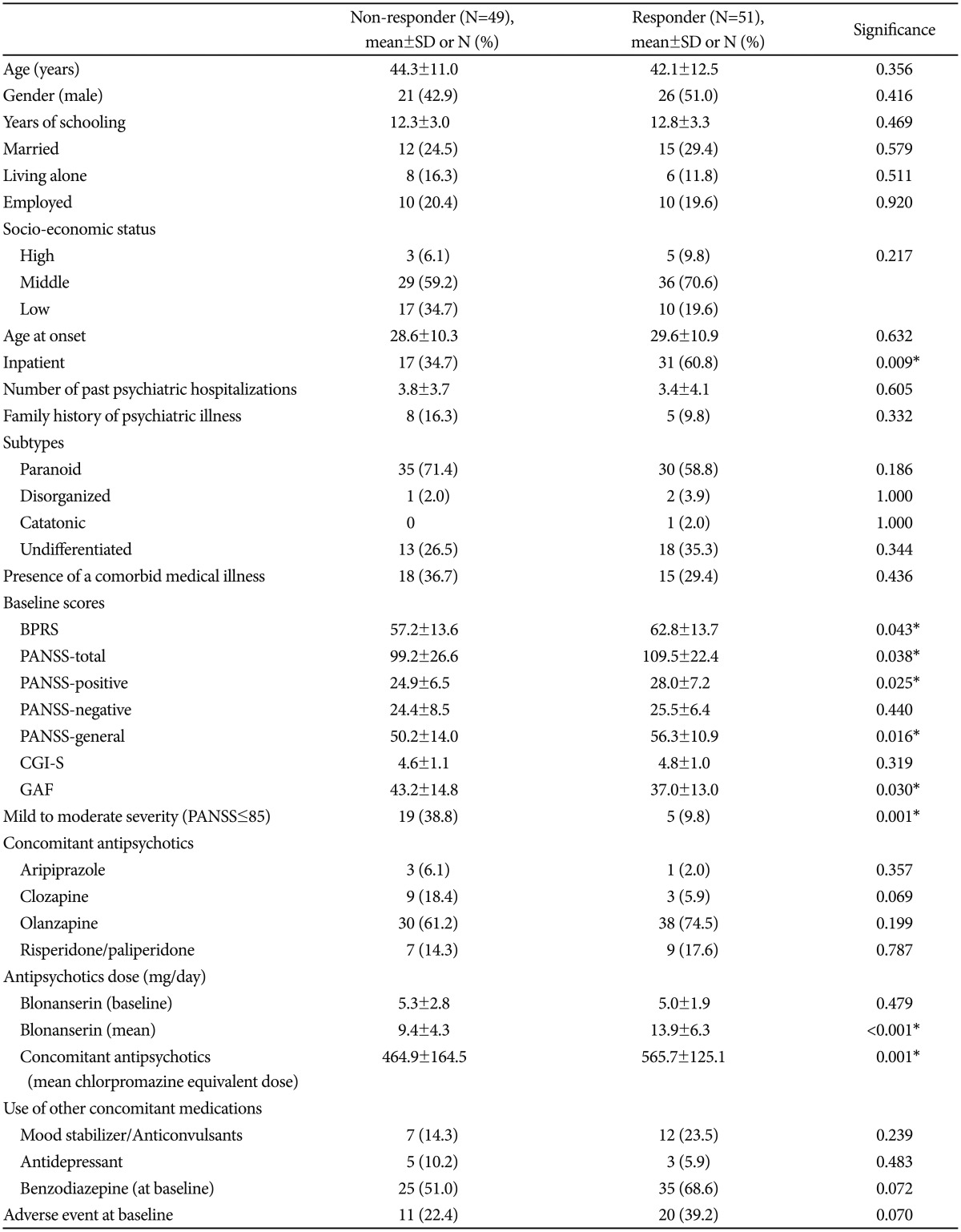
The mean total scores on the PANSS and BPRS significantly decreased from baseline to week 12 (-21.0±18.1, p<0.001 and -14.0±11.6, p<0.001, respectively; Table 2). All three subscales of PANSS (positive, negative, and general psychopathology) also decreased significantly from baseline to endpoint (all p<0.001, Table 2). The CGI-S score decreased from 4.7±1.1, which means 'moderate to markedly ill,' to 3.8±1.1, which means 'mildly to moderately ill.' The number of responders who showed a reduction in PANSS and BPRS scores of 20% or greater at week 12 were 51 (51.0%) and 57 (57.0%), respectively (Figure 2).
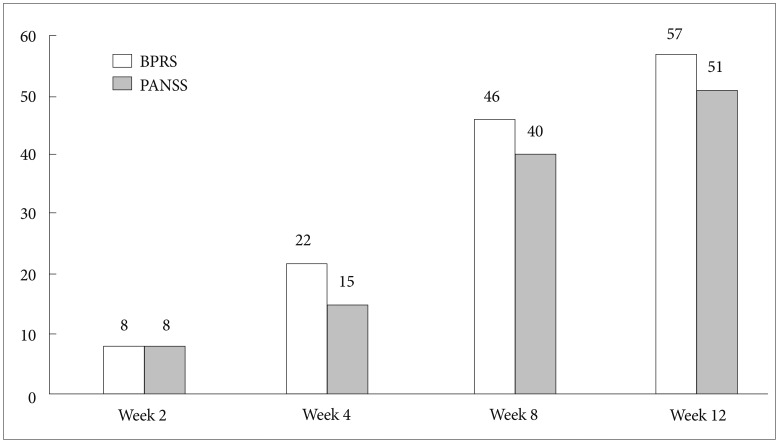
Response rate (≥20% reduction) at each visit (N=100). BPRS: Brief Psychiatric Rating Scale, PANSS: Positive and Negative Syndrome Scale.
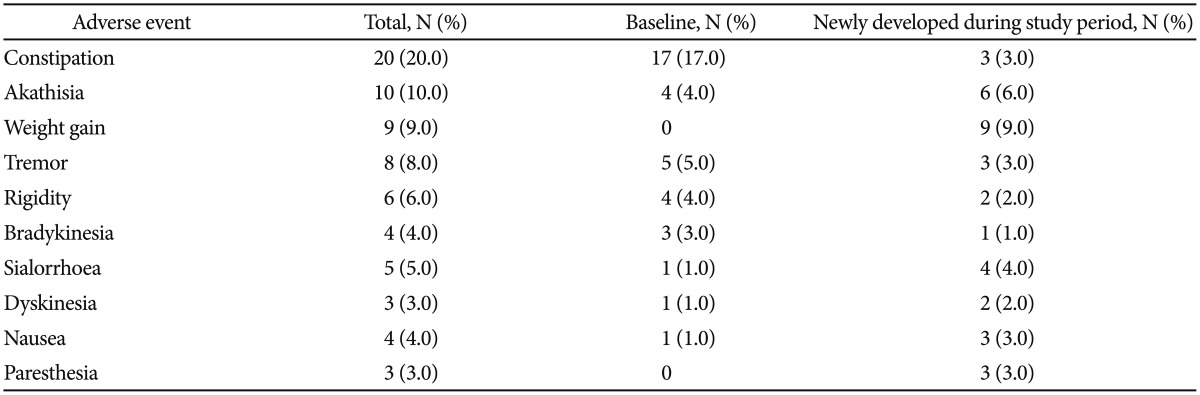
Seventeen participants (17.0%) withdrew from the study prematurely. Six patients (6.0%) were lost to follow-up, 4 patients (4.0%) discontinued due to adverse events (two cases of akathisia, one case of weight gain, and one case of epidural hemorrhage from a fall), two patients (2.0%) due to insufficient response, non-compliance, and/or protocol violation, and one patient (1.0%) discontinued the study by withdrawal of consent. The adverse events frequently reported (≥3%) during the 12 weeks of the study are listed in Table 3. A total of 50 patients (50.0%) reported 225 adverse events. Among them, 41 adverse events in 19 patients were newly developed adverse events or worsening of existing adverse events after blonanserin augmentation was initiated. The severity of every reported adverse event was mild (79.1%, n=178) or moderate (20.4%, n=46), except for one severe case of epidural hemorrhage. The results by RM-ANOVA showed that the scores on SAS (F=1.476, p=0.232), BARS (F=0.400, p=0.672), and AIMS (F=1.204, p=0.305) (Figure 3) were not significantly changed during study period.
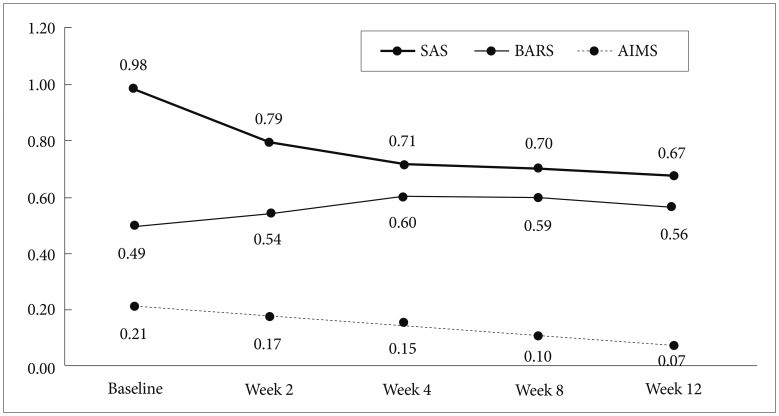
Changes in SAS, BARS, and AIMS scores during study period. SAS: Simpson-Angus Scale, BARS: Barnes Akathisia Rating Scale, AIMS: Abnormal Involuntary Movement Scale.
Blood pressure, body weight, and body mass index (BMI) were compared between baseline and endpoint. Systolic (p>0.999) and diastolic blood pressure (p=0.254) were not significantly different between the two time points (data not shown). Weight significantly increased from 66.7±14.8 kg at baseline to 68.0±15.6 kg at week 12 (p=0.001). Consequently, BMI also significantly increased from 24.6±4.1 kg/m2 to 25.1±4.4 kg/m2. For each concomitant antipsychotics groups, patients treated with blonanserin as an augmentation to olanzapine showed significant increase in weight from 66.3±13.0 kg at baseline to 68.0±14.2 kg at week 12 (p=0.002). There was no significant change in weight during study period in patients treated with other concomitant antipsychotics and blonanserin (data not shown). Ten patients (10.0%) experienced significant (≥7%) weight change during the study period. Among them, 9 patients (8 patients treated with olanzapine and blonanserin, and 1 patient treated with risperidone and blonanserin) gained weight and one patient lost weight.
Characteristics of responders and non-responders
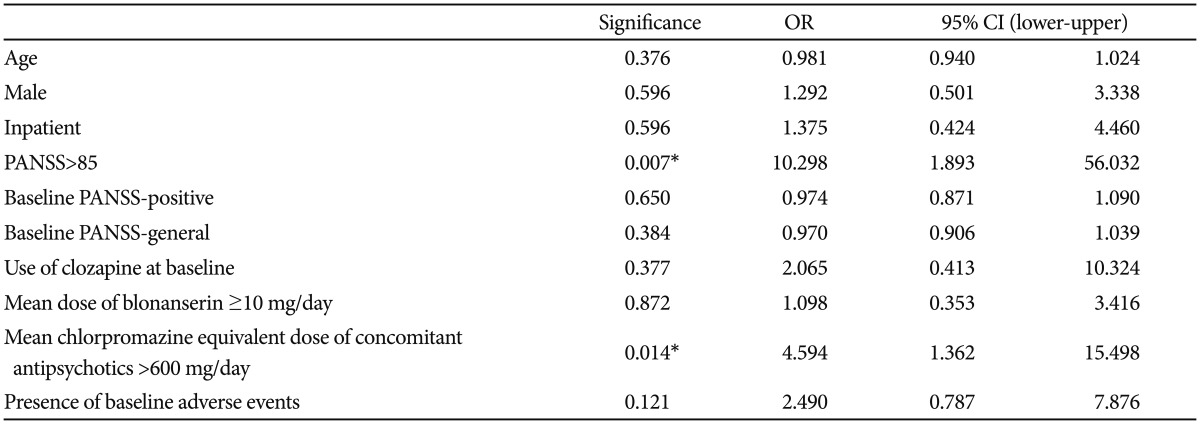
Table 4 shows a comparison of baseline characteristics between people who responded to blonanserin augmentation vs. those who did not. Responders who showed a decrease in their total PANSS score of 20% or more exhibited a higher baseline BPRS total score (p=0.043) and a higher baseline PANSS total score (p=0.038). In addition, responders' scores on the PANSS-positive section (p=0.025) and the general psychopathology (p=0.016) section at baseline were significantly greater than non-responders' scores. Among the responders, 60.8% (n=31) were inpatients, while only 34.7% (n=17) of the non-responders were inpatients (p=0.009). Baseline symptom severity was categorized into mild to moderate symptoms (PANSS total score ≤85) and severe symptoms (PANSS total score >85). Only 9.8% (n=5) of responders exhibited mild to moderate severity, while the majority of responders exhibited severe baseline symptoms. In contrast, 38.8% (n=19) of non-responders had mild to moderate baseline symptoms and only 61.2% of nonresponders had severe baseline symptoms (p=0.001). Reflecting this difference in severity, the mean dose of blonanserin during the study period was 13.9±6.3 mg/day for responders and 9.4±4.3 mg/day for non-responders (p<0.001). Mean chlorpromazine equivalent dose of concomitant AAPs was also significantly different between the two groups (p<0.001); responders were administered 562.2±133.1 mg/day while non-responders took 437.6±193.9 mg/day. However, the starting dose of blonanserin in responders (5.0±1.9 mg/day) was not significantly different from that of non-responders (5.3±2.8 mg/day, p=0.476). Use of other concomitant medication was not significantly different between the groups (Table 4). At baseline, 39.2% (n=20) of responders reported one or more adverse event from their AAP and 22.4% (n=11) of non-responders reported an adverse event from their AAP (p=0.070).
Based on logistic regression analysis with covariates as age, sex, hospitalization, PANSS severity, PANSS-positive score, general subscale score, use of clozapine, dosing for both blonanserin and concomitant AAP (divided at the median value to achieve high and low dose groups), and the presence of baseline adverse events, there were two significant predictors of response (Table 5): severe (PANSS>85) baseline symptoms (OR=10.3, 95% CI=1.9–56.0, p=0.007) and high chlorpromazine equivalent dose (>600 mg/day) of the concomitant AAP (OR=4.6, 95% CI=1.4–15.5, p=0.014).
DISCUSSION
As far as we can tell, this is the first prospective study to investigate the effectiveness and tolerability of blonanserin augmentation in schizophrenic patients. In the present study, blonanserin augmentation improved symptoms in schizophrenic patients who failed to respond sufficiently to treatment with an AAP, especially in patients with severe symptoms and treated with relatively higher dose of the AAP.
There have been only limited studies on the efficacy of augmentation strategies using antipsychotics for schizophrenia. Furthermore, several previous studies suggested only a limited benefit from the augmentation with antipsychotics.3637383940 Some authors1840 suggested that monotherapy in optimum doses already maximizes pharmacologic response, leaving little room for antipsychotic combination for further improvement.
However, in the present study, although the responders (who responded to blonanserin augmentation) were taking 565.7±125.1 mg/day of chlorpromazine equivalent, a dose that nearly corresponds to the upper recommended target range (600 mg/day),35 blonanserin combination afforded additional benefit. The discrepancy between the literature and these findings could be attributed to differences in the study population and treatment regimen. Previous studies that reported a limited benefit from antipsychotics augmentation included mainly treatment-resistant subjects who showed an insufficient response to clozapine3637414243 or had a small sample size of 16–53 patients.394044 Conversely, our study excluded patients who had established treatment-resistance and only 12% of patients were taking clozapine. Furthermore, Correll et al.6 reported that the combination of antipsychotics provided benefits over monotherapy in studies lasting longer than 10 weeks, but not in shorter studies.
We found two significant independent predictors of response to blonanserin augmentation: use of high dose concomitant AAPs and severe baseline psychiatric symptoms. Thus, blonanserin augmentation could be profitable for patients who retain severe symptoms despite compliance with a therapeutic regimen of an AAP. This result is in accordance with meta-analyses by Taylor et al.45 and Correll et al.6 that found significant benefits of the augmentation with a second antipsychotic over a placebo.
However, the results from the present study require interpretation with caution. Because we did not collect data before the start of the intervention, we do not know the characteristics of the patients who were not eligible for the study; for example, several patients discontinued treatment with an AAP before the six-week minimum requirement for enrollment. Our eligibility criteria could have caused selection bias in favor of cases more tolerable to AAP treatment. Moreover, there is a possibility that a low-dose titration regimen could be effective in non-responders. To expound, non-responders could be less tolerable to AAP treatment than responders, and their relative intolerability could cause premature discontinuation of blonanserin, before they had enough time to respond to the blonanserin augmentation. In this situation, non-response may just mean 'intolerability' rather than true 'non-response'. However, two cases of premature discontinuation due to adverse events were reported in both responders and non-responders.
Although there has been a lot of concern regarding the tolerability of antipsychotic combinations,46 only 17% of patients failed to complete the 12-week study. Moreover, premature discontinuation from adverse events was occurred in merely 4% of patients. As reported in a previous review,32 akathisia and extrapyramidal symptoms are frequently reported adverse events. Constipation (20.0%) was the most common adverse event in this study; however, constipation was likely not related to treatment and occurred in patients who were hospitalized (16 of 20 cases) and/or prescribed anti-Parkinsonian agents (17 of 20 cases). Indeed, lack of activity and/or the anti-Parkinsonian agents could have contributed to the constipation.47 Incidence of weight gain in this study (9.0%) during the 12 weeks was relatively higher than a previous study that reported that 3% of participants gained weight gain during 6 weeks of blonanserin augmentation therapy.26 Further long-term data are needed to confirm the association of blonanserin and weight gain.
There are several limitations that should be considered. First, we did not evaluate biochemical metrics such as metabolic parameters (fasting glucose and lipid profiles) and serum prolactin level, nor did we evaluate cognitive functioning, which could have been associated with antipsychotic polypharmacy.30 Second, there was no control group, so the improvement observed could have been a result of the placebo effect or the existing AAP, which can continue to make improvements over the course of 6 months.48 Third, because most of the patients included in this study had been treated with olanzapine (68.0%), results from the present study do not infer effectiveness and tolerability of blonanserin augmentation with other AAPs. In addition, the doses of blonanserin during the study period (11.7±5.8 mg/day) could have been insufficient.
In summary, the results from the present study suggest that blonanserin augmentation might show additional beneficial effect in patients with schizophrenia who showed an insufficient treatment response to AAP monotherapy. However, the results from the present study are insufficient to derive conclusive clinical recommendations. The treatment response was associated with severe baseline psychiatric symptoms and a relatively high baseline AAP dose. Despite the frequent use of antipsychotic polypharmacy in schizophrenia, there is still only limited evidence for its efficacy. Adequately designed, large, double-blind, placebo-controlled, randomized clinical trials and head-to-head comparisons of different AAPs are needed to address the usefulness of antipsychotic polypharmacy in difficult-to-treat schizophrenic patients. These studies need to include a long-term investigation of safety and drug-interactions when combining different AAPs. Furthermore, a further investigation for predictors of response to antipsychotic polypharmacy is needed so that individual cases that will benefit from AAP combinations can be quickly identified.49
Acknowledgments
This study was supported by the Bukwang Pharmaceutical Company, Ltd, Korea. The Bukwang Pharmaceutical Company had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.