Differential Histone Acetylation in Sub-Regions of Bed Nucleus of the Stria Terminalis Underlies Fear Consolidation and Extinction
Article information
Abstract
Objective
The hallmark of anxiety disorders is excessive fear. Previous studies have suggested that selective neural projections from Basal nucleus of stria terminalis (BNST) to amygdala and vice-versa precisely control the fear learning process. However the exact mechanism how the BNST controls fear consolidation and its extinction is largely unknown. In the present study we observed the changes in the BNST sub-regions following fear conditioning and its extinction.
Methods
The change in the number of positive neurons was determined by immunohistochemistry for Acetyl H3 (Histone 3), Acetyl H4 (Histone 4), cAMP response element binding Protein (CBP) and c-fos in three sub-regions of the BNST namely the anterio-lateral BNST (STLP) and anterio-medial BNST (STMA), and lateral-ventral BNST (STLV) of rats subjected to auditory fear conditioning and extinction.
Results
We found significant increase in the number of CBP, acetyl H3 and acetyl H4 positive neurons in the STMA and STLV but not in the STLP after fear conditioning. However, following fear extinction the number of CBP, acetyl H3 and acetyl H4 positive neurons increased significantly in the STLP but not in the STMA and STLV. Similar changes were observed in the number of c-fos positive neurons after fear consolidation and extinction.
Conclusion
The results from this study suggest that the differential histone acetylation in the different sub-regions of the BNST following fear learning and its extinction may be responsible for changes in the neuronal activation patterns resulting in either fear or less fear.
INTRODUCTION
The extended amygdala, comprising of the basolateral nucleus of the amygdala (BLA), central nucleus of the amygdala (CeA), and bed nucleus of the stria terminalis (BNST), play important role in the development of fear and anxiety-like behaviors.1234567891011 The CeA and BNST, project to various anatomic areas involved in the development of fear or anxiety. The fibers from BLA to the BNST pass through CeA and cells in the lateral division of the CeA project to the BNST.12
There is dissociation in the role of the central amygdala (CeA) and the bed nucleus of the stria terminalis (BNST). CeA is involved in the expression of both cued and contextual fear, while BNST is involved in the expression of contextual fear only.1314 The BNST is heterogeneous in structure, and different sub-regions within the BNST appear to make unique contributions to fear and anxiety.1516171819202122 It shares connections with several important emotion-regulating areas in the brain, including the amygdala, dorsal raphe nucleus, hippocampus, hypothalamus, nucleus accumbens, prefrontal cortex, and ventral tegmental area. It directly influences freezing behavior via its projections to the amygdala and periaqueductal gray.2324252627282930313233 Based on cytoarchitecture features, specific neuronal types and their neurochemical make-up, the BNST has been divided into anterio-lateral (BNST-AL/STLP), anterio-medial (BNST-AM/STMA) and anterio-ventral (BNST-AV/STLV) subregions. The hypothalamus-pituitary-adrenal-regulating neurons are concentrated in the ventral (BNST-AV/STLV) and medial (BNST-AM/STMA) portions.34 While the neurons in the dorsolateral part of the BNST-A (BNST-AL/STLP) contribute the outputs to brain stem assemblies regulating fear manifestation.35
Recent reports using techniques that allow selective manipulations of different BNST regions18 or of different cell types within these regions17 show BNST to exert a dual influence over fear expression. On one hand, it maintains contextual fear; on the other, its anterolateral region (STLP), wields an inhibitory influence over fear output networks. The inhibition of STLP neurons results in activation of CeM and increase in the c-fos expression.363738394041 Moreover, Duvarci et al.42 have shown that the increased activity in the BNST during fear conditioning, may be responsible for the decreased cue selectivity in the amygdala. The existence of this dual effect on the fear circuitry has been attributed to regional difference in the activity of BNST-STLP and STMA in relation to learned fear.43 However, the exact molecular mechanism underlying this differential activity in the subdivisions of BNST is not well defined. Recently BNST has also been found to be critical for the reinstatement of extinguished fear after fear extinction.44 Overall in light of these current findings it seems likely that although the BNST plays a major role in context selectivity during fear learning, it also influences the cued learning and its extinction. Till date any direct role of BNST in cued fear consolidation/extinction lies unexplored.
Recent findings suggest that histone acetylation, is an essential and broadly utilized mechanism for fear consolidation, reconsolidation, maintenance, and extinction.4546474849 We speculated that the differential histone acetylation in the sub regions of the BNST might be responsible for the regional differences in the activity observed in BNST leading to differential fear outputs. Pavlovian fear conditioning and extinction in rodents provides a clinically relevant model to explore the behavioral and brain mechanisms operant during fear learning and extinction.50515253
In the present study we explore this possibility by combining auditory fear conditioning/extinction, and analysis of cfos expression patterns as a neuronal activity marker along with that of CBP a histone acetyl transferase (HAT) and acetylation of H3 and H4 in the sub regions of BNST. We gauged the changes in histone acetylation in regulation of differential activity within sub regions of BNST, following fear consolidation and extinction in rats.
METHODS
Animals
Experiments were conducted on SD (Sprague Dawley) rats (250–300 g) housed individually under standard conditions on a 12-h light/dark cycle, temperature maintained at 23℃ and water and food were available ad libitum. Rats were handled for one week before the experiment. All experiments were carried out under strict compliance with Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Environment and Forests, Government of India (Approval number 853/AC/04/CPCSF).
Behavioral procedures
Fear conditioning
After exposing to context A for 3 minutes, rats were fear conditioned in the context A (a transparent Plexiglas chamber with metal grids that was cleaned before each session with 70% ethanol) (V. J. Instruments). Conditioning involved 5 pairings of the Conditioned Stimulus (CS, total duration 10 s, 1 Hz tone, 80 dB) with the Unconditioned Stimulus (US, 1 s foot-shock 0.7 mA, inter-trial interval: 60 s). The US co-terminated with the CS. The freezing was used to measure the conditioned fear response and was defined as cessation of all movement with exception of respiration and non-awake and rest body procedure.5455 Freezing was videotaped and later scored offline by recording the total time spent during 10-second tone CS. In addition freezing was assessed from overhead video tracking device (V.J. instruments, India). The data was converted to freezing values using software where freezing was defined as continuous inactivity lasting for at least 2 seconds. The values were then transformed to freezing percentage and used exclusively to match groups after conditioning. After experiment, animals were replaced back to their home cages. A naïve control group was included which had no exposure to the experimental conditions. Conditioned groups (n=8) were sacrificed 2 hours after experiment.
Fear extinction
Fear conditioning, especially the presence or absence of inhibition or extinction of learned fear,56 is used to study recurring and re-experiencing symptoms of post-traumatic stress disorder (PTSD) in both humans and animal models.5758 One group of conditioned rats (n=8) underwent extinction 24 hours after fear training. Extinction training involved training in the context B (a black-stripped non-transparent Plexiglas chamber with a planar floor that was cleaned before each session with vanilla essence) (V. J. Instrument). Freezing behavior was measured as described above. Animals received 30 presentations of the CS (total duration 10 s, 1 Hz tone, 80 dB, inter-trial interval: 10 s) without presentation of US. After 2 hours extinction, animals (n=8 per group) were sacrificed for immunohistochemical analysis. Another group of age-matched animals that were handled by the experimenter but did not receive any experimental manipulations were used as naive controls in all experiments.
Retention test was performed 24 hours the conditioned and extinction groups along with the control by presentation of the CS (5 tones, total duration 10 s, 1 Hz tone, 80 dB, intertrial interval: 10 s) in context B. The animals, which were used for IHC, did not undergo retention test.
Tissue preparation for immunohistochemistry
Rats were anesthetized with pentobarbital (40 mg/kg, i.p.) and transcardially perfused with chilled normal saline, followed by ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Animals were decapitated and brains were removed and post fixed in 4% paraformaldehyde in Phosphate Buffer for 24 hours and then cryoprotected in 10%, 20% followed by 30% sucrose solution (in 0.1 M phosphate buffer, pH 7.4). Brains were then frozen in at -35℃ for 30 mins and kept at -80℃ until sectioning. Coronal sections 20 µm thick were obtained by sectioning with a cryostat (Microm HM 525, Germany).
Immunohistochemistry
Immunostaining was performed on free-floating 20 µm coronal brain sections containing BNST regions from different groups serially collected for each antibody in order to have matching sections for each antibody from each group (Bregma 0.12 mm up to -0.12, Interaural 9.00 mm). In brief the sections were washed and blocked in PBS containing 1% normal horse serum (NHS Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA, USA), 0.25% tween 20. These brain slices were then incubated overnight at room temperature in anti-Acetyl H3K9, anti-Acetyl H4K5, anti-c-fos and anti-CBP primary antibodies (rabbit monoclonal, 1:1000, 1:500, 1:500, 1:1000 dilution respectively, Abcam) overnight. Sections were then incubated with a biotinylated secondary antibody (anti-rabbit IgG, 1:500 dilution, Vecta-stain Elite ABC kit, Vector Laboratories, Burlingame, CA, USA) for 2 h at room temperature followed by Vecta-Stain Elite ABC kit (Vector Laboratories) followed by DAB staining (DAB peroxidase substrate, Vector laboratories). Sections were mounted and Images of the sections were acquired from at least three sections per rat using the NS-BR image analysis software from Nikon. Expression was analyzed as number of positive nuclei in BNST region of the rat brain. Number of positive neurons was counted using the Nis-BasicResearch image analysis system (Nikon, Tokyo) attached to a Nikon Eclipse Ni microscope (Nikon, Tokyo, Japan).
Statistical analysis
All behavioral data are expressed as means and standard error of the means (±SEM, a measure of the statistical accuracy of an estimate, equal to the standard deviation of the theoretical distribution of a large population of such estimates) and were analyzed with one-way analysis of variance (ANOVA). For each conditioning session, the freezing data were transformed to a percentage of observations. Student t-test was used to compare freezing score. The number of Acetyl H3K9, Acetyl H4K5, CBP and c-fos immuno-positive neurons was analyzed separately in STMA, STLP and STLV using independent one-way ANOVAs for each brain structure. Post-hoc comparisons in the form of Bonferroni tests were performed after a significant overall F ratio.
RESULTS
Fear conditioning and extinction
There was significant increase in the freezing behavior across the conditioning session (p<0.001) with no significant difference in the freezing across the groups (p>0.05). During conditioning significant increase in the level of freezing was observed across the trials and last trial showing robust freezing as compared to first trial (p<0.001, t=30.15) (Figure 1A). After 24 hours the rats underwent fear extinction. During extinction training, all rats exhibited similar attenuation in freezing across the trials and significantly lower freezing was observed in the last trial as compared with the first trial (p<0.001, t=41.44) (Figure 1B). There was significant difference in the % freezing observed between initial and final trials in both the conditioned group as well as extinction group (p<0.001, t=33.74). Freezing was measured in all the control groups (Figure 1C). All control groups had significantly lower freezing compared to the freezing observed in the conditioning group in each trial. Retention test for memory was performed for all groups (Figure 1D). Conditioning group showed robust freezing compared to all groups during retention testing.
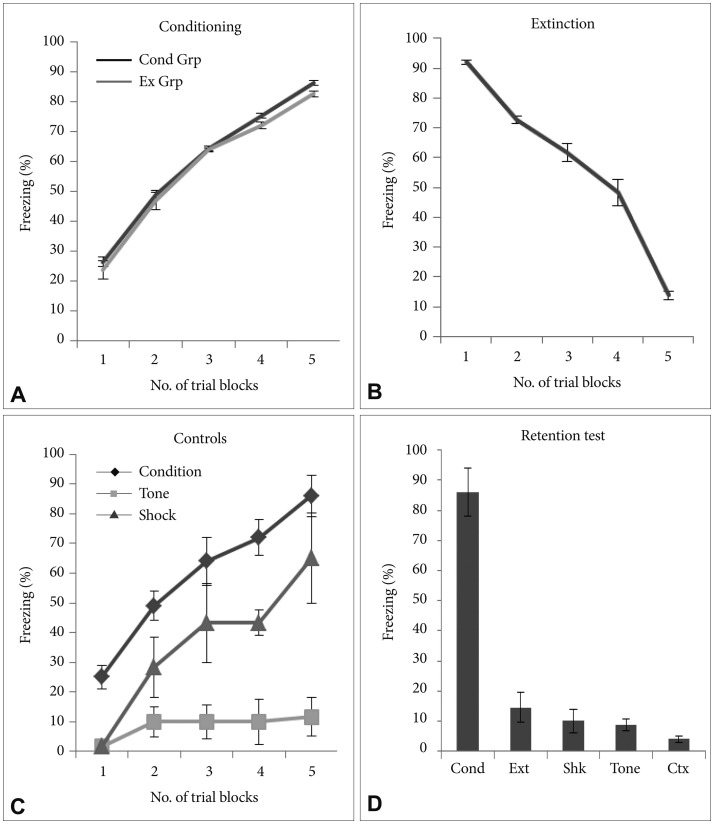
Freezing behavioral. A: During conditioning, there was increase in the freezing behavior both in conditioned as well as extinction groups. B: During extinction training there was attenuation in % freezing across the trials and very low level of freezing was observed in the last trial as compared with the first trial. C: Percent freezing in control groups, shock only and tone only along with conditioned group. D: Percent freezing during retention test across all the groups (*p<0.05). All data are presented as means±SEM (Cond Grp-Conditioning group, Ext Grp-Extinction group, Cond-Condition, Ext-Extinction, Shk-Shock, Ctx-Context).
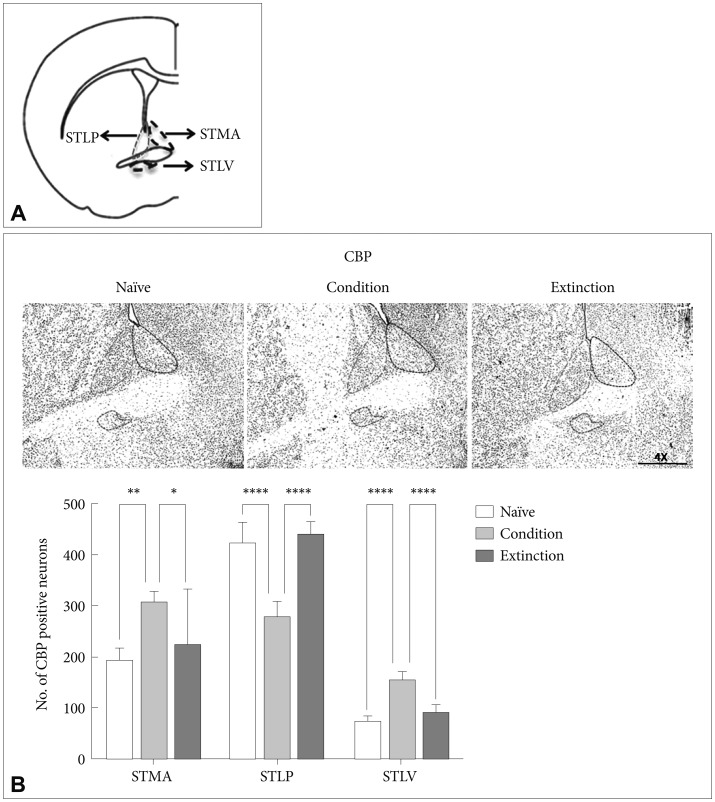
CREB Binding protein (CBP) in the sub-regions of BNST
CBP, a histone acetyl transferase, has been attributed to the active transcription during neuronal activation.59 The number of CBP positive neurons in the sub-regions of the BNST was counted following fear conditioning and fear extinction in rats. Fear conditioning resulted in significant increase in the number of CBP positive neurons in the STMA [p<0.01, F (2, 21)=6.833], and STLV [p<0.0001, F (2, 21)=69.15] but not in the STLP [p>0.05, F (2, 21)=63.81] as compared to the naïve control and extinction group. Similarly there was significant increase in the acetylation of CBP in the STMA [p<0.05; F (2, 21)=6.833] and STLV [p<0.0001; F (2, 21)=69.15]; but not in the STLP as compared to the extinction group. However, extinction of the conditioned fear resulted in significant increase in the level of CBP positive neurons in the STLP [p<0.0001, F (2, 21)=63.81] and significant decrease in the STLV [p<0.0001, F (2, 21)=69.15] as compared to the conditioned group. No significant change was observed in STMA [p>0.05, F (2, 21)=6.833] as compared to condition group. There was no significant change in the STMA (p>0.05) and STLV (p>0.05) as compared to the naïve control (Figure 2B).
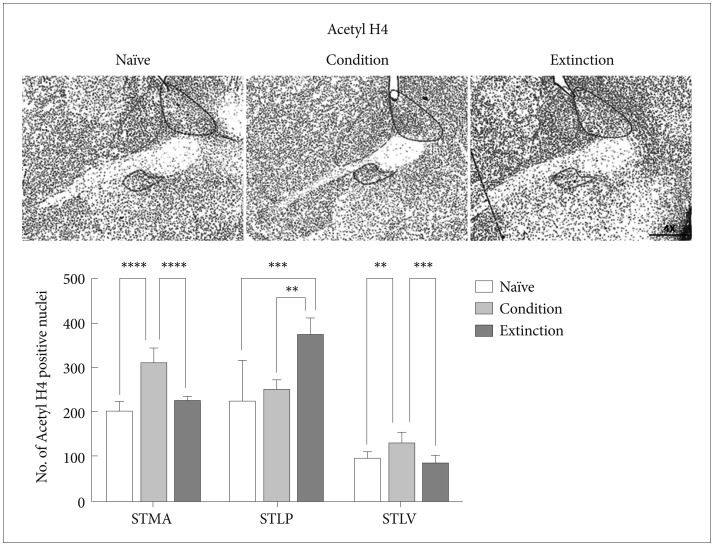
Acetyl H3 and H4 in the sub-regions of BNST
Our next step was to look at the effect of fear consolidation and extinction on Histone H3 and H4 acetylation in these sub-regions (Figure 2A).
Fear memory consolidation led to significant increase in the acetylation of H3K9 in the STMA [p<0.001, F (2, 21)=12.10] and STLV [p<0.01, F (2, 21)=11.46] with no significant changes in the STLP as compared to the naïve control (p>0.05). Similarly there was significant increase in the acetylation of H3K9 in the STMA [p<0.01; F (2, 21)=12.10] and STLV [p<0.01, F (2, 21)=11.46]; but not in the STLP as compared to the extinction group. However extinction of the fear resulted in significant elevation in the number of acetyl H3K9 positive neurons in the STLP [p<0.0001, F (2, 21)=47.31] when compared to the condition group/naïve control and there was no significant change in the number of acetyl H3K9 in the STMA and STLV following extinction as compared to the naïve control (Figure 3).

Represents Acetyl H3 positive neurons expression in BNST region following conditioning and extinction (STLP-BNST lateral-posterior, STLV-BNST lateral-ventral, STMA-BNST medial-anterior).
The change in the acetyl H4K5 level was similar to that of acetyl H3K9. Conditioning training resulted in increase in the acetyl H4 positive neurons in the STMA [p<0.0001, F (2, 21)=47.18] and STLV [p<0.01, F (2, 21)=12.83] region of the BNST in compare with naïve control but no significant change was observed in the STLP (p>0.05). The extinction of the conditioned fear resulted in increased Acetyl H4 level in the STLP [p<0.001, F (2, 21)=12.52] region of the BNST but not in the STMA (p>0.05) and STLV (p>0.05) region as compared to the naïve control (Figure 4). Similarly extinction of the conditioned fear resulted in increased Acetyl H4 level in the STLP [p<0.01, F (2, 21)=12.52] region of the BNST as compared to the conditioned group and significant decrease in the STMA (p<0.0001)and STLV (p<0.001) region as compared to the conditioned group. Extinction group shows significant changes with conditioned group in H4K5 level in STLP region [p<0.05, F (2, 21)=12.52].
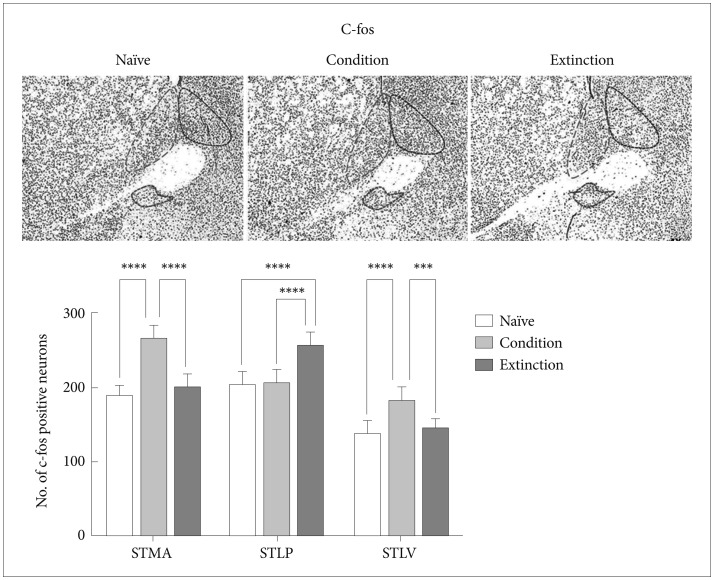
c-fos in the subregions of BNST
As it is well known that there is a strong correlation between transcriptional activity and acetylation of the histone H3 and H4 lysine residues,60 we next looked at c-fos, an immediate early gene and a marker for neuronal activity in a region. It was found that the increase/decrease in the level of c-fos in the STMA, STLV and STLP of BNST following fear conditioning and its extinction was similar to the H3/H4 acetylation and paralleled to changes in the level of CBP, Acetyl H3 and Acetyl H4. Conditioning resulted in increase in the number of c-fos positive neurons in the STMA [p<0.0001, F (2, 21)=41.56] and STLV [p<0.0001, F (2, 21)=12.83] but not in the STLP. Extinction resulted in significant increase in the number of c-fos positive neurons in the STLP [p<0.0001, F (2, 21)=12.52] but not in the STLV and STMA as compared to the naïve control (Figure 5). Similarly extinction of the conditioned fear resulted in increase in the no. of c-fos positive neurons in the STLP [p<0.0001, F (2, 21)=19.88] region of the BNST as compared to the conditioned group and significant decrease in the STMA (p<0.0001) and STLV (p<0.001) region as compared to the conditioned group. Extinction group shows significant changes with conditioned group in c-fos level in STLP region [p<0.001, F (2, 21)=12.52].
DISCUSSION
The present study was undertaken to shed light on how that different sub-regions of BNST respond differentially to the fear memory consolidation and its extinction. Interestingly, there was region specific activation in the BNST sub regions following fear consolidation and extinction. The differential activity as evident by the changes in the expression of the immediate early gene c-fos paralleled to the changes in the histone (H3/H4) acetylation patterns in these regions.
Fear conditioning model used in the present study is an extensively used model for associative learning in rats.61 The freezing levels in both the conditioned and extinction groups are consistent with earlier studies. There was consequent increase and reduction in % freezing observed in the present study during fear acquisition and extinction training respectively.62636465 The conditioning group retained maximum freezing while the extinction group as well as naïve control retained least fear levels when tested 24 hours after the training.
The BNST is a collection of nuclei and different BNST regions form contrasting connections.22 The ventral BNST-AV/STLV and medial BNST-AM/STMA portions have mainly hypothalamus-pituitary-adrenal-regulating neurons69 while BNST-AL/STLP neurons contribute most BNST outputs to brain stem structures regulating fear expression.6667 In the present study we investigated the role of BNST, in regulating fear learning and extinction. We looked at the changes in STMA, STLP and STLV of the BNST through immunohistochemistry for CBP, Acetyl H3/H4 and c-fos following fear conditioning and extinction. These sub-regions showed different neuronal activation patterns after fear conditioning and extinction. The heterogeneous projections from amygdala and the intrinsic BNST network especially the GABArgic inputs from CeA and glutamatergic inputs from BLA6869 to these regions exert opposite influences on fear response. A recent study has shown that the projections from STLP to STMA and STLV are purely inhibitory. On activation the STLP via these inhibitory projections tends to reduce the activity in STMA and STLV neurons. On other hand a reduction in STLP activity results in positive feedback effect where disinhibition from STMA to STLP inputs increase the return inhibitory connections leading to further disinhibition of STMA neurons.70 Our findings indicate that following fear learning the activity is higher in STMA and STLV but not in STLP as evident by the c-fos positive neurons. Similarly following extinction there was an increase in the activity of STLP neurons but not of STMA and STLV. This may be due to the reciprocal inhibitory control of these sub-regions on each other.
Regulation of chromatin structure is one of the fundamental molecular mechanisms contributing to long-term memory formation. The stabilization of normal and pathological fear memories involves distinct phases that are dependent on regionally and temporally distinct epigenetic mechanisms.717273 It has also been suggested that the functional and mechanistic outcomes of epigenetic marks differ according to the brain region and the stage of memory formation. In this study we found that the neuronal activation paralleled to the changes in histone acetylation and also to that of CBP, a histone acetyl transferase. CBP and p300 are large multidomain proteins that possess intrinsic histone acetyltransferase (HAT) activity. It has been shown that CBP is critical for the in vivo acetylation of lysines on histones H2B, H3, and H4. CBP's homolog p300 is unable to compensate for the loss of CBP function in knockouts.7475 Glen Schafe's group has shown that cued fear conditioning is associated with increased expression of acetylated histone H3 in the lateral amygdala.76 Moreover acetylation of lysine 5 on H4 increases in the hippocampal region following contextual fear conditioning.77
The STMA and STLV were active during conditioning and the STLP following extinction training. Several studies have shown that stimuli, which usually induce activity-dependent gene transcription, lead to an increase in histone acetylation at the c-fos promoter as well as other activity-regulated gene promoters.7879 To date, the majority of studies examining the requirement of these HATs in memory processes have focused on p300/CBP using knock-out models in the context of hippocampal-dependent memory paradigms, including object recognition, spatial memory, and contextual fear memory.8081828384858687 In the present study we observed the histone acetylation pattern in sub regions of BNST following conditioning and extinction.
Overall, there was increase in the level of CBP, Acetyl H3/H4 and c-fos in the STMA following fear conditioning as compared to the naïve control. This is consistent with earlier reports which have shown that the CS responsiveness of the STMA neurons is similar to that of CeM,8889 both having innervations from prelimbic cortex (PL), a region playing an active role in fear acquisition.9091 However, there was an increase in the levels CBP, Acetyl H3/H4 and c-fos in the STLP but not in the STMA and STLP following extinction as compared to the conditioned group and naïve control. It is well known that the activation of STLP neurons, which are mostly GABAergic, results in inhibition of CeA. In earlier studies by our group and others it has been shown that extinction results in inhibition of the CeA neurons.92 It has also been shown through electrophysiological studies that potentiation of GABAergic neurons of STLP,19 results in startle enhancement due to the inhibition of STLP neurons and consequent disinhibition of CeA. This means that the inhibition and dis-inhibition of STLP has an important role to play in fear response and fear extinction. Our results suggest towards the existence of such a mechanism where the STLP is acting as a buffer controlling both the freezing as well as the inhibition of CeA.9394 Overall there was increase in the acetylation of H3/H4 in STMA during conditioning and in STLP during extinction are in line with earlier studies which have shown that the STLP and STMA exert opposite influence on fear output network.22 Our observation that correlated the acetylation levels to the neuronal activation in STMA, STLV following conditioning and in STLP following extinction which may be indirectly responsible for the opposite effects.
In this study the histone acetylation paralleled to region specific neuronal activation in the BNST following fear memory consolidation and its extinction. The changes observed following fear consolidation and extinction may be the outcome of differential activity in the sub-regions of the BNST, which in turn may be under epigenetic control. CBP, a HAT, enhanced the acetylation of H3 and H4 and the neuronal activation as evident by c-fos levels positively correlated to the Histone acetylation. In other words the epigenetics might be playing an important role in buffering the behavioral outcomes by differentially controlling the expression and thus the neuronal activation in the sub-regions of the BNST. Our findings represent the first comprehensive look at the role of CBP/HAT activity in BNST-dependent learning and memory and associated synaptic plasticity, and make an additional contribution toward our understanding of the role of epigenetic mechanisms in the regulation of memory and synaptic plasticity in the mammalian brain.