Neuroprotection in Schizophrenia and Its Therapeutic Implications
Article information
Abstract
Schizophrenia is a chronic and debilitating mental disorder. The persisting negative and cognitive symptoms that are unresponsive to pharmacotherapy reveal the impairment of neuroprotective aspects of schizophrenia. In this review, of the several neuroprotective factors, we mainly focused on neuroinflammation, neurogenesis, and oxidative stress. We conducted a narrative and selective review. Neuroinflammation is mainly mediated by pro-inflammatory cytokines and microglia. Unlike peripheral inflammatory responses, neuroinflammation has a role in various neuronal activities such as neurotransmission neurogenesis. The cross-talk between neuroinflammation and neurogenesis usually has beneficial effects in the CNS under physiological conditions. However, uncontrolled and chronic neuroinflammation exert detrimental effects such as neuronal loss, inhibited neurogenesis, and excessive oxidative stress. Neurogenesis is also a major component of neuroprotection. Adult neurogenesis mainly occurs in the hippocampal region, which has an important role in memory formation and processing. Impaired neurogenesis and an ineffective response to antipsychotics may be thought to indicate a deteriorating course of schizophrenia. Oxidative stress and excessive dopaminergic neurotransmission may create a vicious cycle and consequently disturb NMDA receptor-mediated glutamatergic neurotransmission. Based on the current evidences, several neuroprotective therapeutic approaches have been reported to be efficacious for improving psychopathology, but further longitudinal and large-sample based studies are needed.
INTRODUCTION
Generally, neuroprotection is defined as a mechanism to maintain homeostasis and the functional integrity of the central nervous system (CNS) against any neurodegenerative and neurotoxic insults.1 Practically, neuroprotection can also refer to an intervention that helps to restore the functional integrity of the brain in response to neurobiological stress.1 Neuroprotection can be facilitated by intrinsic compensatory mechanisms and external treatment approaches. The failure of compensatory neuroprotective processes results in various anato-mical and functional impairments in the CNS, including schizophrenia.
Schizophrenia is a debilitating and severe mental illness that affects approximately 1% of the general population worldwide.2 Schizophrenia is characterized by positive, negative, and cognitive symptoms with poor insight and impaired psychosocial function.3 Although acute psychosis is controlled with pharmacotherapy, negative and cognitive symptoms tend to be unresponsive to pharmacotherapy.456 In addition, most second-generation antipsychotics (SGA) except clozapine have been shown to have no significant advantages over first-generation antipsychotics (FGA) even in the domain of negative and cog-nitive symptoms.7 The chronicity and deterioration of the illness raise the possibility that schizophrenia may have neurodegenerative aspects.
Etiological associations with prenatal exposure to infection,89 obstetric complications,10 and genetic vulnerabilities1112 support the notion that schizophrenia has a neurodegenerative etiology. In addition, cortical gray matter loss has been shown to be associated with childhood-onset schizophrenia.13 Gray matter loss appears to be slowly progressive, and findings support the underlying neuroanatomical basis of a deteriorating course of the disease. Moreover, functional hypofrontality during wo-rking memory-related tasks has been found in the dorsolateral prefrontal cortical region in schizophrenia.14
The above considerations suggest that neurobiological vulnerability and impaired neuroprotection greatly contribute to the etiopathology of schizophrenia. In this article, we reviewed current evidences suggesting the association between neuroprotection and schizophrenia in the etiological and therapeutic perspectives.
NEUROINFLAMMATION AND NEUROGENESIS OF SCHIZOPHRENIA
Inflammation has been considered to be a causative factor in schizophrenia, which has been shown to involve a dysregulated cytokine system. Cytokines are pleiotropic glycoproteins produced by both peripheral immune cells and glial cells in the brain.1516 Cytokines usually have an important role in mediating immune signals and inflammatory processes in the peripheral system and in the brain; cytokines also are involved in various neural interactions such as neurogenesis and synaptic plasticity.1718 In this regard, neuroinflammation is distinguished from systematic inflammation. Particularly, pro-inflammatory cytokines such as interleukin-1β (IL-1β), interleu-kin-6 (IL-6), and tumor necrosis factor-α (TNF-α) primarily mediate and facilitate neural activities as well as inflammatory processes. In particular, during the early period of disease development, activated pro-inflammatory cytokines may exert detrimental effects on the brain. There is mounting evidence that prenatal exposure to pro-inflammatory cytokines induces impaired spatial memory, neuronal loss, and gliosis in the hippocampus.19 Moreover, neurodevelopmental injuries due to excessive pro-inflammatory cytokines increase susceptibility to schizophrenia.202122
Neurogenesis is defined as a coordinating process of generating new neurons from neural stem cells.23 Since the first report from animal studies24 and human postmortem studies of the hippocampal region,25 numerous studies have focused on adult neurogenesis. Neurogenesis consists of many steps including stem cell proliferation, neuronal differentiation, migration, and ultimately, integration of newly generated neurons into functional neuronal circuitry.26 Adult neurogenesis occurs in specific regions including the subventricular zone (SVZ) in the lateral ventricle and the subgranular zone (SGZ) in the dentate gyrus of the hippocampus. Generally, adult hippocampal neurogenesis is significantly and positively associated with cognitive function such as learning and memory function, which are mainly processed in the hippocampus.2728293031 Several studies have shown that newly-generated neurons in adulthood have a role in synaptic plasticity and cognitive functions in psychiatric diseases including depression and schizophrenia.263032 To date, the most widely investigated psychiatric illness in terms of neurogenesis is probably major depressive disorder, although the pathophysiological link is still uncertain.333435 Importantly, pervasive cognitive dysfunction that does not adequately respond to antipsychotics is a distinguishing clinical characteristic of schizophrenia.363738 In fact, there is a possibility that specific molecular and cellular dysfunction in the hippocampus contributes to the development of schizophrenia.39 Additionally, decreased hippocampal volume and activity are the most consistent findings of neuroimaging studies of schizophrenia.4041 One previous study has revealed that impaired prefrontal-hippocampal connectivity is associated with impaired spatial working memory in schizophrenia.42 In addition, in a postmortem study of human psychiatric patients, neural stem cell proliferation in the dentate gyrus was only impaired in patients with schizophrenia, but not in those with depression.43 Another postmortem study revealed that the number of dividing cells is decreased in the SGZ of patients with schizophrenia.44 Collectively, molecular and neuroanatomical evidence supports the notion that hippocampal adult neurogenesis is closely associated with schizophrenia.
Interestingly, neurogenesis is closely associated with pro-inflammatory cytokines and neuroinflammation. Pro-inflammatory cytokine receptors are highly aggregated in regions associated with cognitive functioning such as the hippocam-pus.4546 Pro-inflammatory cytokines and their receptors may inhibit neurogenesis and impair cognitive function.4748 Numerous reports have supported the idea that several pro-inflammatory cytokines affect neurogenesis.
IL-1β has been consistently shown to be associated with neurogenesis in brain, especially in the hippocampal region.4950 It is of note that the inhibition of IL-1β blocks the decrease in neurogenesis caused by acute stress, as well as the anhedonic and antineurogenic effects of chronic stress.49 A previous study revealed that sustained hippocampal IL-1β expression has a detrimental effect on adult neurogenesis.51 IL-1β is also known to be involved in the IFN-γ-induced suppression of neurogenesis.52 Leukemia inhibitory factor (LIF) is also an important cytokine involved in schizophrenia. LIF shares glycoprotein 130 (gp130) with other IL-6 cytokine family members.53 LIF mainly modulates neuronal activity such as glial cell activity, the inflammatory process, transcription pathways, and neurogenesis.545556 An appropriate amount of LIF is essential for normal neuronal function. In an animal study, LIF knock-out mice showed decreased astrocyte and microglial cell activities compared to wild type mice.54 Various insults to the CNS may induce over-expression of LIF, which may lead to neurobehavioral abnormalities similar to schizophrenia.56 Additionally, LIF gene polymorphism is associated with susceptibility to schizophrenia and working memory deficit.57
Another cytokine, TNF-α, has an important role in neuronal activity in the CNS.5859 TNF-α is usually maintained at low level under normal physiological conditions.60 However, when the microenvironment in the CNS is altered by injuries such as physical head trauma, infection, and ischemic attack, TNF-α is activated by glial cells.6162 Interestingly, TNF-α has a pivotal role in mediating neurogenesis via its receptors, tumor necrosis factor receptor (TNFR)-1 and TNFR-2. Those two receptors are different in several aspects. First, TNFR-1 contains an intracellular ‘death domain’ and is involved in detrimental effects such as neuronal apoptosis and neurodegeneration, whereas TNFR-2 is usually involved in neuroprotection.6364 Thus, TNFR-1 and TNFR-2 are differentially involved in proliferation and differentiation of hippocampal neural stem cells. TNFR-1 exerts a negative influence on hippocampal adult neurogenesis,656667 whereas TNFR-2 is involved in promoting neurogenesis.6869 TNF-α is also closely associated with synaptic plasticity by inducing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors that mediate glutamatergic activity.70 Because human neural stem cells have both TNFR-1 and TNFR-2, whether neurogenesis is enhanced or inhibited depends on the microenvironment in the CNS and the context of insults.
OXIDATIVE STRESS AND MITOCHONDRIAL DYSFUNCTION IN SCHIZOPHRENIA
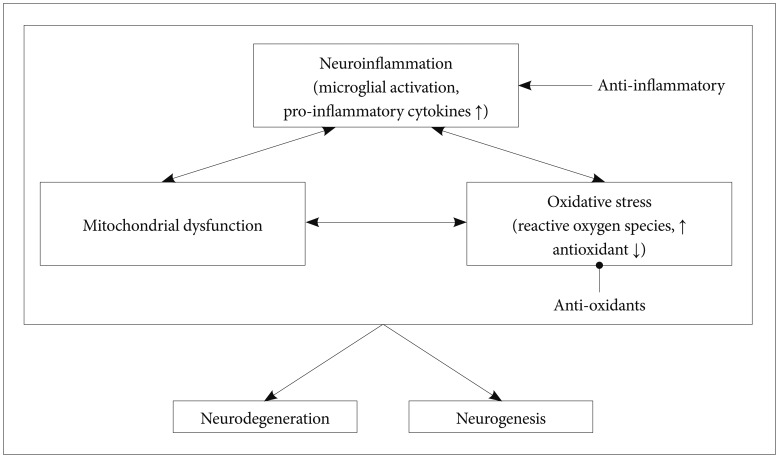
Neuroinflammation is closely associated with excessive oxidative stress.7172 The CNS is more vulnerable to oxidative stress than are other organs. The brain consumes large am-ounts of oxygen, lacks anti-oxidative compounds, has a high ratio of membrane surface area to cytoplasmic volume, has a high concentration of polyunsaturated fatty uric acids, and contains a large amount of metals.73747576 There have been many evidences suggesting that neuroinflammation and oxidative stress have close interactions and play an important role in the pathophysiology of schizophrenia.777879 Reactive oxygen species (ROS), which include superoxide, nitric oxide, and hydrogen peroxide, are highly reactive molecules, and free radicals are naturally created as byproducts of electron transport and energy metabolism. Appropriate duration and amount of ROS activity contribute to enhanced cellular functioning.80 However, an imbalance between amounts of ROS and antioxidant system products can result in oxidative damage.81 An imbalance between oxidative stress and antioxidants is also demonstrated in schizophrenia.8283 Several studies have reported excessive activity of oxidants84 and decreased antioxidants in patients with schizophrenia.85
Antioxidants consist of enzymatic and non-enzymatic materials. Enzymatic antioxidants include superoxide dismutase (SOD), catalase, and glutathione dismutase. Non-enzymatic antioxidants are albumin, uric acid, bilirubin, vitamin C, vitamin E, and β-carotene. In particular, glutathione plays a major role in the redox process.86 Glutathione exerts various effects in the CNS including directly detoxifying drugs, ROS, and electrophilic xenobiotics; storing cysteine; promoting neurodevelopment; and enhancing excitatory glutamatergic neurotransmission.878889
The possible actions by which a deficit of glutathione contributes to the development of schizophrenia may be through NMDA receptors. Dysregulation of dopaminergic neurotransmission and hypofunction of NMDA receptor activity are regarded as the core mechanisms of schizophrenia.90 Under normal physiological conditions, glutathione enhances NMDA receptor-mediated glutamatergic neurotransmission. Many animal studies have reported that glutathione exerts an agonistic effect on NMDA receptors.8791 However, in the presence of uncontrolled and persistent chronic oxidative stress, glutathione level is decreased and leads to NMDA receptor dysfunction.9293 Deficits in glutathione level are associated with neurological deficits in various neuropsychiatric diseases.94 Glutathione regulates redox-sensitive sites such as the NMDA receptor. Further, a key glutathione-synthesizing enzyme, glutathione cysteine ligase modifier (GCLM), is decreased under oxidative stress.95 GCL dysregulation and deficit glutathione inhibit the detoxification of oxidative stress that results from dopamine metabolism and consequently lead to neural injury, especially in dopamine neuron-rich regions.9697 Glutathione is reduced in the prefrontal cortex and striatum,949899100 which are closely associated with the psychopathology of schizophrenia. Many studies have shown that a deficit in glutathione is associated with psychopathology and clinical variables in schi-zophrenia.100101102
Mitochondrial dysfunction, which is closely associated with oxidative stress, is also associated with neurodegenerative change in schizophrenia. Mitochondria are mainly involved in producing energy via the electron transport chain during the process of oxidative phosphorylation. Mitochondria also regulate intracellular calcium homeostasis and apoptosis. Neurons require the large amount of energy that is mainly produced by the mitochondrial respiratory system in order to maintain the ion gradient between the extracellular and intracellular membranes. Several studies have reported that genetic and structural abnormalities of mitochondria are associated with various psychiatric diseases.103104 In particular, psychosis and cognitive deterioration are common features of mitochondrial diseases such as mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome.105
Mitochondrial metabolism is also important for producing ROS. In particular, mitochondrial DNA is vulnerable to oxidative stress because it is located in the mitochondrial inner membrane, where a substantial amount of ROS is produced.106 Mitochondrial injury and oxidative stress form a vicious cycle. Mitochondria injured by excessive oxidants produce a greater amount of ROS, which leads them to be more vulnerable to oxidative stress.107 For example, an overload of oxidative stress causes lipid peroxidation. Lipid peroxidation of the mitochondrial membrane produces toxic molecules such as 4-hydroxynonenal and malondialdehyde, which in turn disturb mitochondrial membrane fluidity.108109 The impaired mitochondrial membrane leads to dysregulation in ionic balances and excessive intracellular calcium influx, which consequently causes neuronal death and impairs synaptic plasticity.110111112
NEUROPROTECTIVE AND ANTI-INFLAMMATORY THERAPY IN SCHIZOPHRENIA
Randomized controlled trials have investigated the efficacy of adjuvant COX-2 inhibitor on antipsychotics treatment in schizophrenia. Of the four extant studies, three have reported significant improvement in psychopathology,113114115 whereas one study showed no significant efficacy of COX-2.116 An improvement in psychiatric symptoms with anti-inflammatory treatment may be associated with the duration of illness. Wh-ereas the three studies finding a significant therapeutic efficacy of the COX-2 inhibitor were based on patients with a short illness period,113114115 the one study with a negative result was conducted with patients with relatively chronic schizophrenia.116 A meta-analysis showed that augmentation of anti-inflammatory drugs to the antipsychotics would be a potential therapeutic options in patients with schizophrenia, although more evidences should be accumulated.117
Several antioxidant treatment approaches have reported that antioxidants are effective for improving psychopathology in schizophrenia. Because glutathione is not bioavailable, it is not possible to able to be directly used. Instead, N-acetyl cysteine (NAC), which is a glutathione precursor, has been used for schizophrenic patients. A double-blind, placebo-controlled study demonstrated that NAC (2 g/day) ameliorated negative symptoms, but not positive symptoms in patients with chronic schizophrenia.118 Another double-blind, placebo-controlled study revealed that NAC improved mismatch negativity, which is associated with NMDA receptor function, in patients with schizophrenia.119
Several studies have consistently reported that ginkgo biloba augmented with haloperidol improves negative symptoms and increases SOD level in patients who experience chronic hospitalizations and treatment resistance due to schizophrenia.120121122 A meta-analysis revealed that ginkgo as an add-on treatment to antipsychotics significantly improved total and negative symptoms in patients with chronic schizophrenia.123
Several studies have reported that schizophrenia is associated with low serum vitamin C levels.124125126 A previous double-blind, placebo-controlled study showed that a vitamin C (500 mg/day) supplement with atypical antipsychotics (olanzapine 10 mg/day, quetiapine 200 mg/day, or ziprasidone 40 mg/day) significantly decreased serum malondialdehyde (MDA) level and psychopathology compared to a placebo add-on group.127
Antipsychotics have been thought to have anti-inflammatory properties. In a meta-analysis of patients with acute relapse or first onset schizophrenia, levels of peripheral pro-inflammatory cytokines including IL-6, IL-1β, and IFN-γ were significantly decreased after antipsychotic treatment compared to baseline.128 Those results are in line with previous studies reporting that both typical and atypical antipsychotics suppress microglial activation, thus inhibiting inflammatory mediators.129130131132133
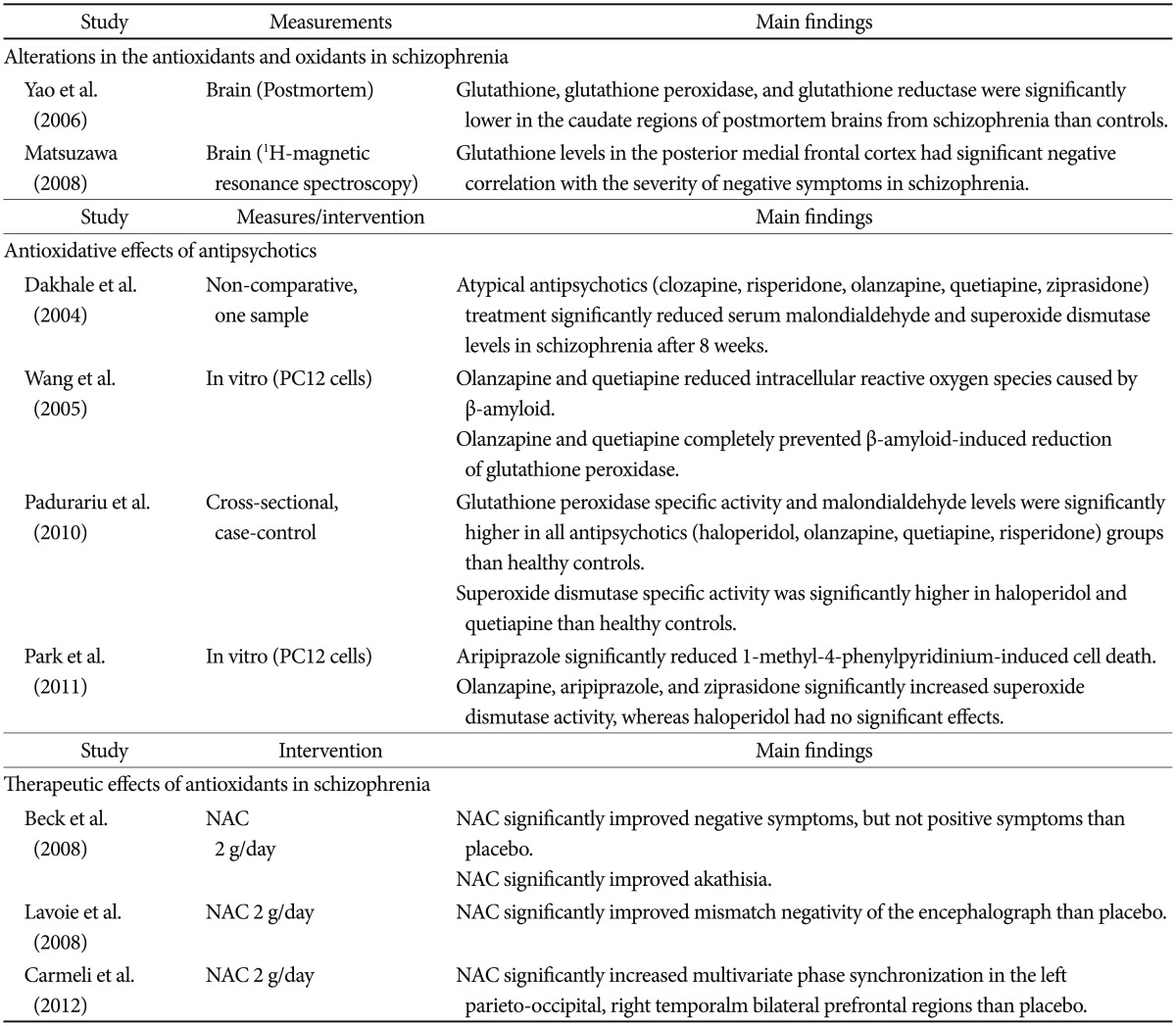
Antipsychotics also enhance neurogenesis. In one study, chronic treatment with a low dose of clozapine (0.5 mg/kg) increased the number of bromodeoxyuridine (BrdU)-positive cells in the dentate gyrus 24 hours after BrdU administration, whereas haloperidol (0.05 or 2 mg/kg) or a high dose of clozapine (20 mg/kg) had no significant effect.134 Interestingly, the increased BrdU-positive cells with a low dose of clozapine could not survive or integrate into existing hippocampal circuitry. These intriguing results suggest that antipsy-chotics influence neuronal proliferation, but not survival. It has been speculated that the limitations of the effect of antipsychotics on neurogenesis may be associated with an insufficient treatment response in terms of negative and cognitive symptoms, as well as a deteriorating course of schizophrenia. At the same time, antipsychotics demonstrate antioxidant effects.135136137138 One study group has consistently reported that initial SOD level in schizophrenia was lower than that in controls, and both typical and atypical antipsychotics significantly increase SOD level.72139140141 The improvement in the psychopathology of patients with schizophrenia has been correlated with the degree of change in SOD level. Although the mechanisms by which antipsychotics exert antioxidant effects still have not been clearly identified, differences between typical and atypical antipsychotics appear insignificant.135141 The aforementioned evidences supporting the relationships between oxidative stress and schizophrenia were summarized in Table 1.
CONCLUSION
Current evidence clearly supports a close link between neuroprotection and the onset and symptoms of schizophrenia. Several factors affecting neuroprotection, which include neuroinflammation, neurogenesis, and oxidative stress, appear to increase vulnerability to schizophrenia (Figure 1). However, there are several issues in the field of neuroprotection in schizophrenia. First, the measurements of pro-inflammatory cytokines, oxidative stress markers, and neural cell proliferations may not suitable for distinguishing schizophrenia from other neuropsychiatric diseases. Second, despite several clinical findings supporting the effectiveness of adjuvant antioxidant and anti-inflammatory agents, further longitudinal studies and studies with a large sample are needed. Third, the he-terogeneous subtypes of schizophrenia should also be con-sidered limiting factors of schizophrenia-specific findings.