Efficacy and Safety of Bitopertin in Patients with Schizophrenia and Predominant Negative Symptoms: Subgroup Analysis of Japanese Patients from the Global Randomized Phase 2 Trial
Article information
Abstract
Objective
The aim of the present study was to perform a subgroup analysis of data from a phase II global, multi-center, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of bitopertin, a glycine reuptake inhibitor that activates N-methyl-D-aspartate receptors by increasing the concentration of glycine in the synaptic cleft, in Japanese and non-Japanese patients with schizophrenia and predominant negative symptoms.
Methods
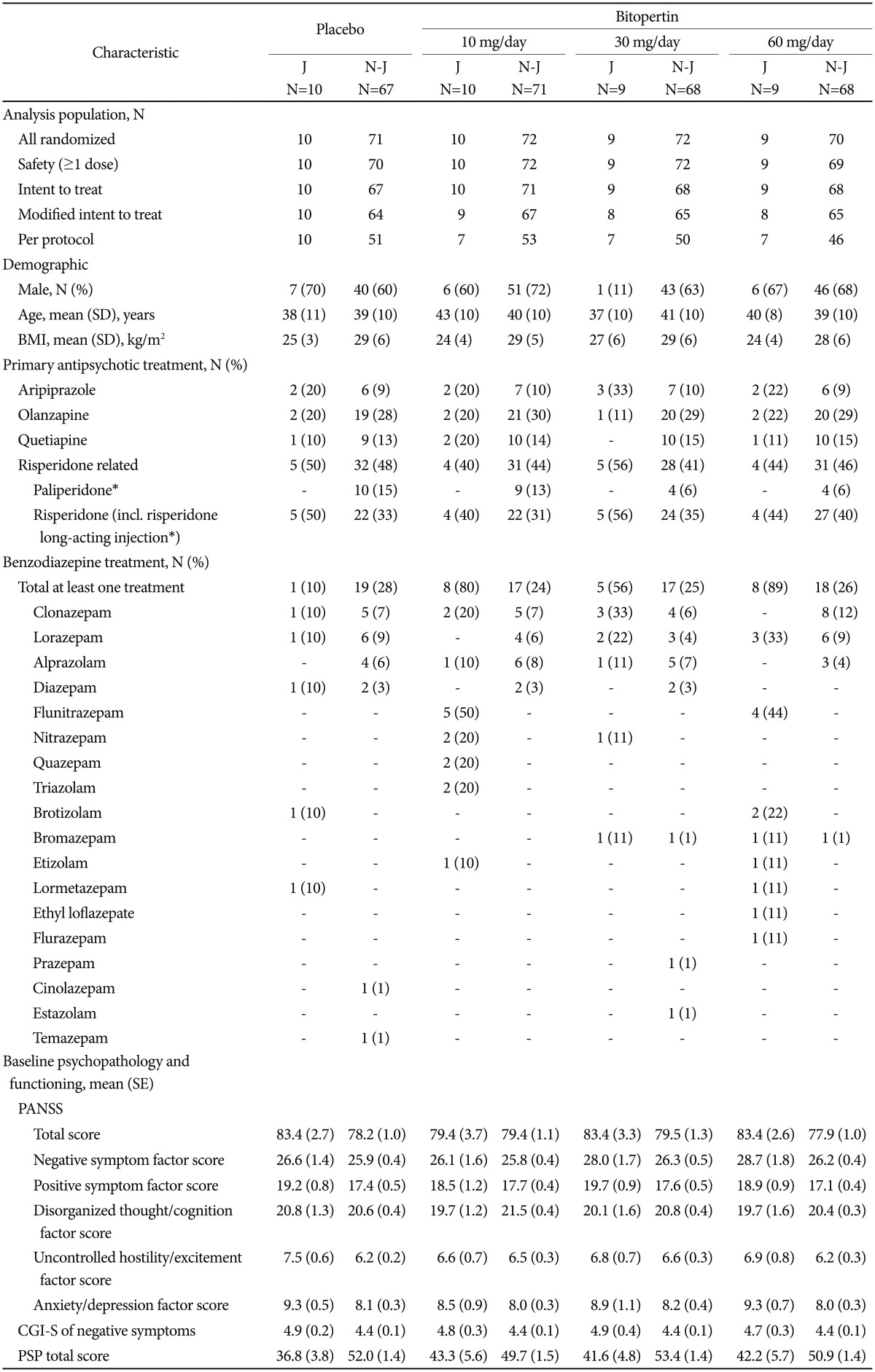
Patients with schizophrenia and predominant negative symptoms on one or two antipsychotic drugs, including atypical antipsychotic drugs (olanzapine, risperidone, quetiapine, aripiprazole, and paliperidone) as the primary treatment, received bitopertin (10, 30, or 60 mg/day) or placebo once daily for 8 weeks as an add-on treatment. Efficacy was assessed using the Positive and Negative Syndrome Scale (PANSS) negative symptom factor score (NSFS).
Results
The efficacy of bitopertin (10 mg and 30 mg) was similar between Japanese and non-Japanese patients. In the bitopertin 60-mg group, no difference from the placebo group was observed in Japanese or non-Japanese patients. The response to placebo was lower in Japanese patients, and there was a trend towards a greater difference in the change in PANSS NSFS between the placebo group and the 10-mg and 30-mg groups among Japanese patients. The safety profile of bitopertin was favorable in Japanese and non-Japanese patients.
Conclusion
According to this subgroup analysis from a global phase II study of bitopertin, there was no difference in terms of efficacy and safety between Japanese and non-Japanese patients.
INTRODUCTION
Patients with schizophrenia experience positive symptoms (e.g., hallucinations, delusions, catatonic symptoms, and incoherence of thought), negative symptoms (e.g., social withdrawal and blunted affect), and cognitive deficits. Reportedly, negative symptoms are closely related to functional impairments regarding the maintenance of personal care, performance of household chores, learning, ability to work or attend school, and interacting with others.12 Additionally, these symptoms seem to have a greater impact on social functioning than do positive symptoms.3
Conventional antipsychotic drugs do not provide sufficient improvement in negative symptoms; thus, effective treatments to satisfy this unmet need are anticipated.45 Because N-methyl-D-aspartate receptor (NMDAR) inhibitors, such as phencyclidine and ketamine, induce not only positive but also negative symptoms and cognitive deficits in healthy adults,6789 NMDAR dysfunction has been proposed as a factor contributing to the negative symptoms of schizophrenia.10 Therefore, NMDA enhancers, such as glycine reuptake inhibitors (GRIs), may improve negative symptoms.
Bitopertin, a GRI that selectively inhibits a glycine transporter, GlyT1, activates NMDAR by increasing the concentration of glycine in the synaptic cleft.11 A proof-of-concept, global phase II study was conducted to investigate the clinical effect of bitopertin on negative symptoms in patients with schizophrenia.12 A significant improvement was observed in the 10-mg and 30-mg groups compared with the placebo group in the per-protocol population, and also a trend toward improvement was observed in the intent-to-treat (ITT) population.
To the best of our knowledge, this was the first global study in the field of psychiatry, led by Europe and the US and involving Japan. Therefore, examining the impact of the differences between Japanese and non-Japanese patients based on demographic characteristics and social environment on the evaluation of the efficacy and safety of bitopertin may have clinical relevance. This manuscript is a report of a subgroup analysis comparing the efficacy and safety of bitopertin in 38 Japanese patients with those in non-Japanese patients.
METHODS
Study design
This is a subgroup analysis of data from a global, multi-center, randomized, double-blind, placebo-controlled study conducted between February 2008 and September 2009, including the participation of ten countries: Austria, Brazil, France, Germany, Hungary, Mexico, Poland, Russia, the US, and Japan. The patients were randomized to treatment in Japan and elsewhere separately. The detailed methods were previously published.12
Study subjects
According to the recommendations by the National Institute of Mental Health-Measurement and Treatment Research to Improve Cognition in Schizophrenia consensus statement on negative symptoms,1314 schizophrenic outpatients with chronic stable positive symptoms and prominent negative symptoms were recruited, and patients with uncontrolled extrapyramidal symptoms and depressive symptoms were excluded from the present study. All patients gave informed consent to participate in the study and their anonymity was preserved.
Drugs
Bitopertin (10, 30, or 60 mg/day) or placebo was administered once daily, in addition to the primary treatment (one or two antipsychotic drugs, including atypical antipsychotic drugs such as olanzapine, risperidone, quetiapine, aripiprazole, and paliperidone).
Endpoints
The primary endpoint was the mean change from baseline in the negative symptom factor score (NSFS) of the Positive and Negative Syndrome Scale (PANSS).15 The secondary endpoints were the PANSS NSFS response rate (≥20% change), other PANSS factor scores, PANSS total score, PANSS symptom scores, Clinical Global Impression-Improvement (CGI-I) of negative symptoms,16 functioning on the Personal and Social Performance scale (PSP),17 and safety.
Statistical methods
A mixed-model repeated-measure analysis (MMRM) was used for variables with repeated scheduled measurements. The MMRM model included fixed-effect terms for treatment group, visit week (categorical and nested within patient), baseline score, treatment-by-visit interaction, and the interaction between baseline score and visit week. Statistical methods are described in detail in the original paper.12 Data from Japanese and non-Japanese sites for key efficacy and safety variables were compared in the ITT population. This comparison should be interpreted with caution because of the small number of patients from Japanese sites. Statistical significance was not evaluated. In a post-hoc analysis, the correlations between the PSP total score and the score for each PANSS symptom factor at baseline were examined.
RESULTS
In the original study, 323 of 477 screened patients were randomly assigned to receive placebo or bitopertin (10, 30 or 60 mg/day). Results have been published and other details can be found in the original paper.12
Patient demographics and baseline characteristics
Of the 323 patients randomly assigned to treatment, 38 were Japanese: of these, all 38 were included in the ITT population and 34 patients completed the study (Figure 1). Regarding primary antipsychotic treatment, the most commonly used drug was risperidone (47%), followed by aripiprazole (24%) and olanzapine (18%) in Japanese patients. In non-Japanese patients, risperidone-related products were the most common [risperidone, paliperidone, and risperidone long-acting injection, (45%)], followed by olanzapine (29%), quetiapine (14%), and aripiprazole (9%). The proportion of Japanese patients receiving benzodiazepine concomitantly was higher than that of non-Japanese patients (58% versus 26%, respectively) (Table 1).

CONSORT diagram. *some patients were excluded for more than one reason. J: Japanese, N-J: non-Japanese.
Efficacy
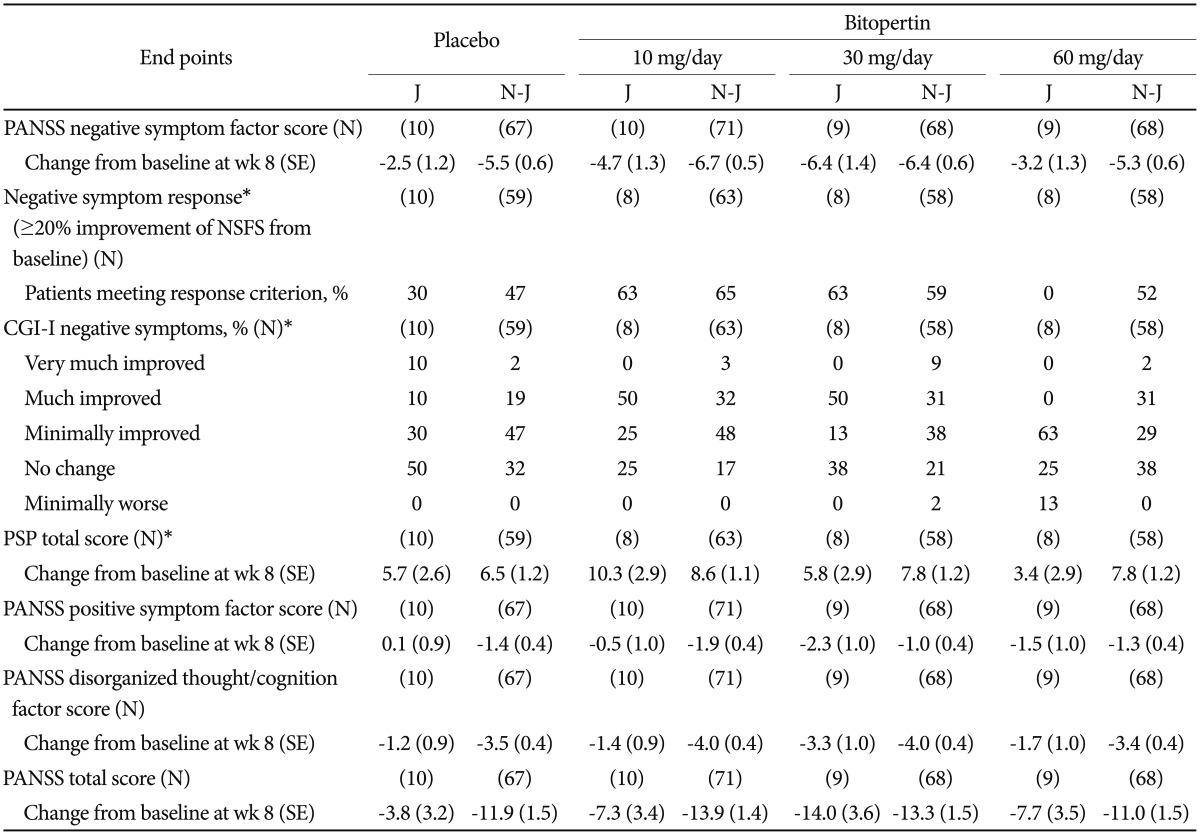
At week 8 from baseline, an effect of bitopertin 10 mg and 30 mg on negative symptoms was observed in both Japanese (−4.7 and −6.4 in the 10-mg and 30-mg groups, respectively) and non-Japanese patients (−6.7 and −6.4, respectively) on the basis of a greater difference in the change in PANSS NSFS (Table 2). The same trend was observed for PANSS NSFS response rate in both Japanese and non-Japanese patients (Table 2, Figure 2). There was no difference in the change from baseline at week 8 in PANSS NSFS between the bitopertin 60-mg group and the placebo group in Japanese (−2.5 and −3.2 in the placebo and 60-mg groups, respectively) or non-Japanese patients (−5.5 and −5.3 in the placebo and 60-mg groups, respectively), which was in congruence with the results of the original study (Table 2). In Japanese patients, the response to placebo was lower than in non-Japanese patients, and there was a trend toward a greater difference in the change in PANSS factor scores between the placebo group and the 10-mg and 30-mg groups, in particular for the 30-mg group (Figure 3, Table 2). In the 10-mg and 30-mg groups, the effect sizes (the mean difference divided by the common standard deviation) for the change in NSFS were −0.60 and −1.02, respectively, among Japanese patients, and −0.25 and −0.19, respectively, among non-Japanese patients (Figure 4).

Positive and Negative Syndrome Scale negative symptom factor score response rates at week 8 (intent-to-treat population).
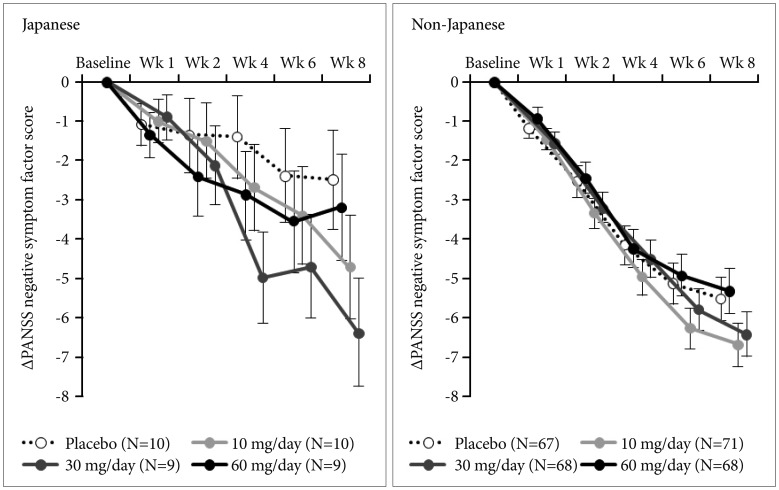
Change in Positive and Negative Syndrome Scale (PANSS) negative symptom factor score from baseline [intent-to-treat population; mean (standard error)].

Differences from placebo and effect sizes of Positive and Negative Syndrome Scale negative symptom factor score change from baseline at week 8 (intent-to-treat population) based on mixed-model repeated-measure analysis.
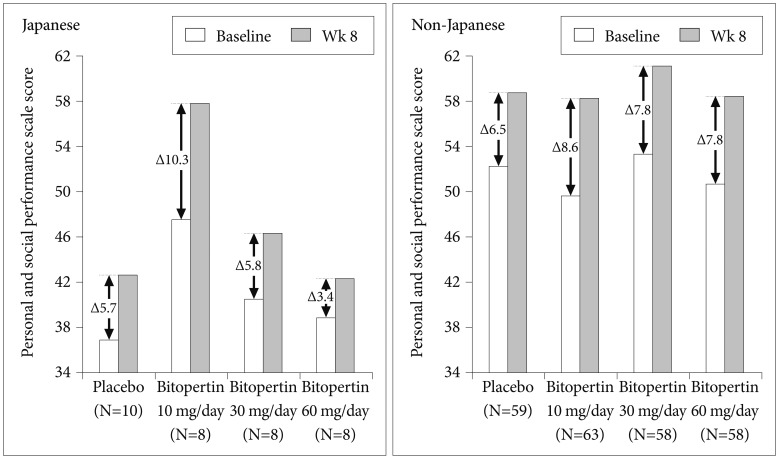
The PSP score improved at week 8 in both Japanese and non-Japanese patients, while baseline PSP scores were generally lower in Japanese than non-Japanese patients (Figure 5, Table 2).
The response rate (“Very much improved” and “Much improved”) in CGI-I negative symptoms score at week 8 (Figure 6, Table 2) was greater in the 10-mg and 30-mg groups than in the placebo group, in Japanese and non-Japanese patients.
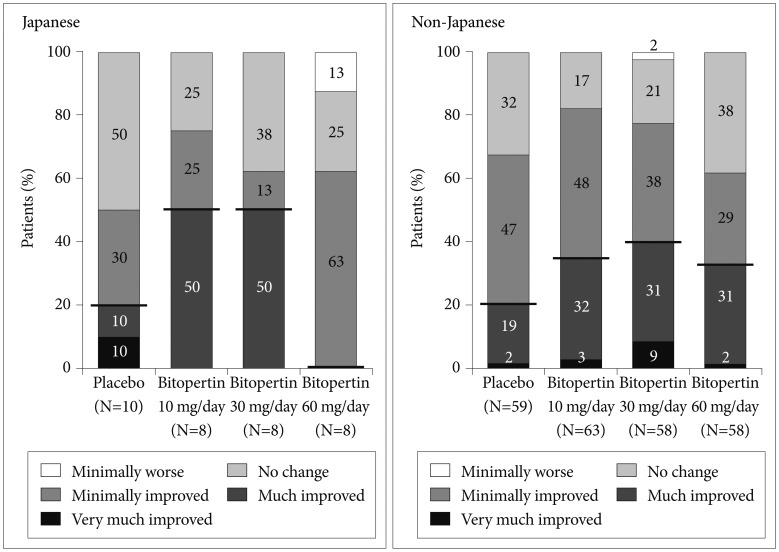
Change in Clinical Global Impression-Global Improvement negative symptoms at week 8 (intent-to-treat population).
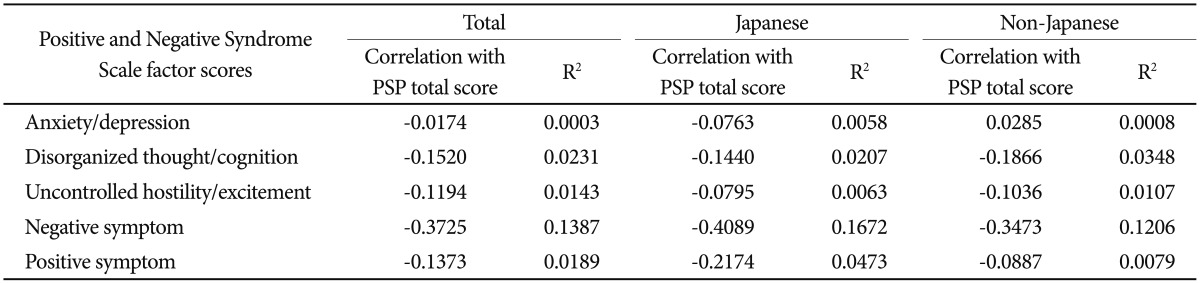
In a post-hoc analysis, the correlations between the PSP total score and the score for each PANSS symptom factor at baseline were examined. The strongest correlations were between the PSP score and the PANSS negative symptom factor score, in both Japanese and non-Japanese patient populations (Japanese: correlation coefficient=−0.41; non-Japanese: correlation coefficient=−0.35) (Table 3).
Safety
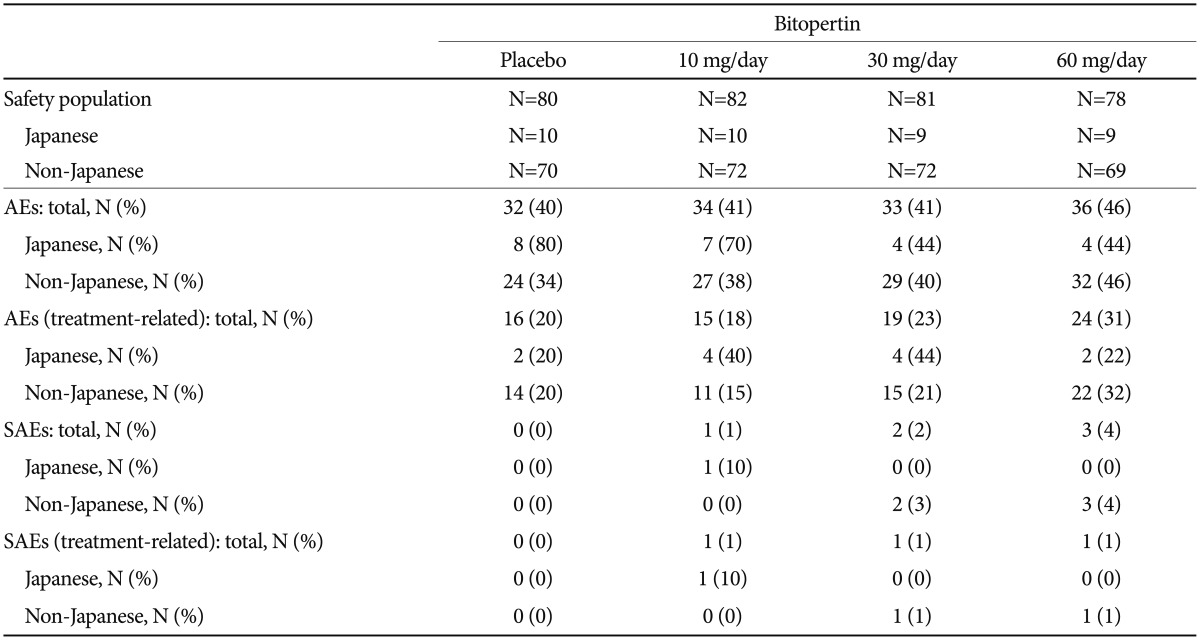
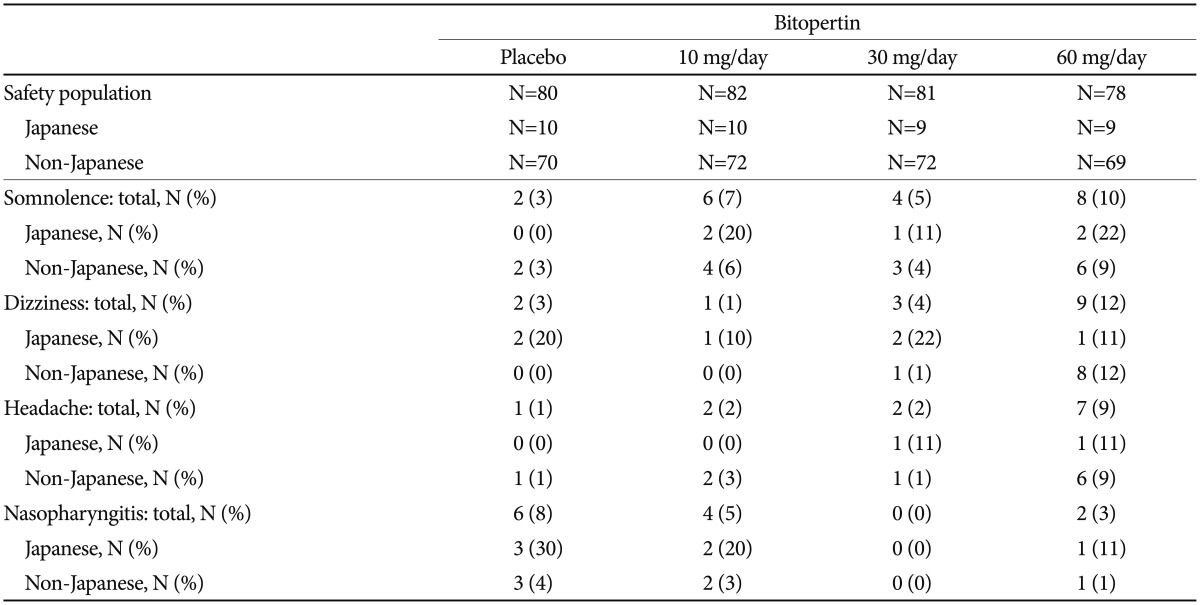
There were no major differences in safety profiles between Japanese and non-Japanese patients (Table 4 and 5). The most common adverse event in Japanese patients was “dizziness” and “Nasopharyngitis”. The incidence rates of “somnolence,” “dizziness,” and “headache” were higher in the 60-mg group in non-Japanese patients, but this trend was not observed in Japanese patients (Table 5). “Anxiety” in one patient in the 10-mg group was the only serious adverse event observed among Japanese patients (data not shown).
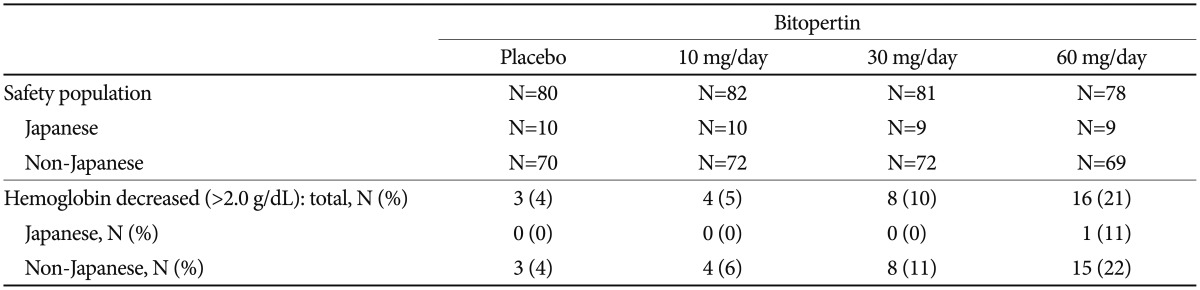
Dose-dependent changes in the mean blood hemoglobin concentration were observed in Japanese and non-Japanese patients. Decrease of >2.0 g/dL in hemoglobin was seen in 4 to 22% of the non-Japanese patients; however, only one Japanese patient was reported in the 60-mg group (Table 6). Otherwise, no clinically significant changes were observed in vital signs, electrocardiograms, or extrapyramidal symptoms (BAS, SAS, and AIMS) in the safety analysis population. No other particular concerns were observed in Japanese patients.
DISCUSSION
This clinical trial was a proof-of-concept study designed to assess the efficacy of bitopertin on negative symptoms of schizophrenia in clinical settings. In this study conducted in ten countries, including Japan, a significant improvement in PANSS NSFS, the primary endpoint, was observed in the per-protocol population. The safety profile of bitopertin was also confirmed in patients with schizophrenia. Furthermore, in this study, a similar profile was observed in the Japanese patients treated with bitopertin 10 and 30 mg; the 60-mg dose was essentially ineffective for improving PANSS NSFS.
Although baseline PSP scores were lower among Japanese patients, there were no differences between Japanese and non-Japanese patients in terms of demographic characteristics and baseline symptom scores on the PANSS and CGI. Social acceptance of patients with schizophrenia has been reported to be lower in Japan than in the US and Australia.1819 According to the PSP, which assesses the severity of impairment of personal and social functioning based on information from caregivers, prejudice toward patients with schizophrenia may be a contributing factor to the lower rating in Japanese patients among those with similar severity of symptoms. Examination of differences between Japanese and non-Japanese patients in each PSP domain revealed that the personal and social functioning tended to be rated lower on average in the Japanese population in all domains of “Socially useful activities,” “Personal and social relationships,” “Self-care,” and “Disturbing and aggressive behaviors” (data not shown). Despite the difference in the baseline, changes in PSP were similar between Japanese and non-Japanese patients.
Although differences were observed in the type of antipsychotic drugs and concomitant use rate of benzodiazepine between Japanese and non-Japanese patients, these did not significantly affect the efficacy and safety evaluations. Risperidone-related products were found to be the most commonly used concomitant drugs in both Japanese and non-Japanese patients. Olanzapine and aripiprazole were also commonly used in both Japanese and non-Japanese patients. However, olanzapine was much more commonly used among non-Japanese patients (29% vs. 18%), and aripiprazole was much more commonly used among Japanese patients (24% vs. 9%). The World Federation of Societies of Biological Psychiatry Guidelines for Treatment of Schizophrenia recommends olanzapine and amisulpride for the treatment of primary negative symptoms.20 A meta-analysis conducted by Leucht et al.21 has shown that aripiprazole is the best tolerated and has the least sedative effects among the atypical antipsychotics allowed as concomitant baseline medications in the present study (risperidone, olanzapine, quetiapine, and aripiprazole). The common use of aripiprazole in the Japanese patient population may be a reflection of prescription practices that put emphasis on the tolerability in chronic patients rather than the treatment of primary negative symptoms. The difference between Japanese and non-Japanese patients in the proportion of patients receiving a benzodiazepine concomitantly in the present study appears to reflect the general trend of prescription use for patients with schizophrenia in Japan.
In the present study, the placebo response was lower in Japanese patients than in non-Japanese patients. Similar trends were observed in clinical trials on another antipsychotic drug for schizophrenia.22232425 In the original paper, the authors suggested the improvement observed in the placebo group may be attributed to the intense interaction and intimate communication between the patients and the study staff in a clinical trial setting.12 In Japan, patients enrolled in clinical studies are mostly those cared for by clinical investigators. Therefore, there are no major differences between the routine clinical practice and that during participation in a clinical study. This may explain the small placebo response in this study.
A meta-analysis of placebo-controlled studies of antipsychotic drugs conducted from 1970 onwards found a gradual increase in placebo response in the past 2–30 years.26 The authors suggested this increase could be explained by an increase in the number of study sites per study and a decrease in the percentage of academic sites participating. In the editorial accompanying the meta-analysis,27 Leucht et al. discuss that nonacademic sites are more motivated by financial incentives; thus, they aim to enroll as many patients as possible. This trend will create greater variation in study results, thereby decreasing differences in efficacy between the active drug and placebo. There were 13 facilities participating in this study from Japan, and most of them are academic sites (nine university hospitals and three national hospitals). This participation proclivity may have contributed to the difference in placebo response between Japanese and non-Japanese patients.
To minimize the risk of differences in technical expertise and to increase inter-rater reliability, raters in this study received standardized training. Absolute differences were observed in all treatment arms, which showed that appropriate evaluation was implemented. Additionally, cultural, habitual, and environmental factors were considered possible explanations for the placebo response rate difference. Although these factors cannot be ruled out, the mean change in Japanese patients was not lower than that in non-Japanese patients in all bitopertin groups, while the mean change in the PANSS positive symptom factor score in the 30-mg group was actually lower in non-Japanese than in Japanese patients.
There were no major differences in the safety profile between Japanese and non-Japanese patients, and bitopertin was generally well tolerated. No adverse events were reported with higher incidences among Japanese patients. No abnormal laboratory changes characteristic of Japanese patients were reported. These results show that bitopertin is tolerated in Japanese and non-Japanese patients with schizophrenia and predominant negative symptoms.
Glycine is essential for heme synthesis in erythroid progenitors and reticulocytes,28 and is taken up by GlyT1, into the cells.29 Therefore, there is concern that bitopertin, which is a GlyT1 inhibitor, may decrease hemoglobin. As expected, a dose-dependent decrease was observed. Although the number of patients with a decrease of >2.0 g/dL in blood hemoglobin concentration increased in a dose-dependent manner among non-Japanese patients, this decrease was observed in only one Japanese patient in the group receiving the highest dosage. However, there was no difference in the mean blood hemoglobin change between Japanese and non-Japanese patients.
Concordantly with the original study,12 this subgroup analysis for Japanese patients confirmed the efficacy of bitopertin on negative symptoms as an add-on to the existing antipsychotic therapy, and its favorable safety profile in patients with schizophrenia, during an 8-week treatment. When comparing Japanese and non-Japanese patient populations, the efficacy and safety profiles of bitopertin were generally similar in the 10-mg and 30-mg groups. Although there were differences between Japanese and non-Japanese patients in the type and frequency of antipsychotic drugs and concomitant benzodiazepine use, none of these differences affected the efficacy and safety evaluations significantly.
Acknowledgments
The authors thank Dr Jennifer Dreyer and Dr Keyra Martinez Dunn for providing medical writing support, which was funded by Chugai Pharmaceutical. Co., Ltd. The authors would also like to thank F. Hoffmann-La Roche for conducting the statistical analyses. The authors are grateful to Dr. Daniel Umbricht and Dr. Dragana Bugarski-Kirola for their critical review of the paper. This study was sponsored by F. Hoffman-La Roche Ltd and Chugai Pharmaceutical Co., Ltd.
We thank the investigators of the phase 2 study for recruiting and assessing patients: Csaba Almasi, Aleksander Araszkiewicz, Alla Avedisova, Istvæn Bitter, Przemyslaw Bogacki, Rodrigo Bressan, David Brown, Miranda Chakos, Læszl Csekey, Marek Cwiakala, Daniel Dassa, Natalia Dobrovolskaya, Helio Elkis, Tetsuro Enomoto, Donald Garcia, Hamilton Grabowski, Andrey Gribanov, Jun Ishigooka, Nakao Iwata, Mieczyslaw Janiszewski, Lala Kasimova, Hiroaki Kawasaki, Mary Knesevich, Sændor Koffler, Alex Kopelowicz, Alexsander Kotsubinsky, Jelena Kunovac, Tamas Kurimay, Toshihide Kuroki, Ichiro Kusumi, Mark Lerman, Andreas Mahler, Margarita Morozova, Joaquim Mota Neto, Tadashi Murakami, Judit Nagy, Henry Nasrallah, Nikolay Neznanov, Claus Normann, Mark Novitsky, Yohtaro Numachi, Toshinari Odawara, Tetsuro Ohmori, Irismar Reis de Oliveira, Alfonso Ontiveros, György Ostorharics-Horvæth, Hiroki Ozawa, Høctor Pinedo, Philippe Raymondet, Robert Reisenberg, Sandra Ruschel, Margot Schmitz, Gunther Schumann, Kazumasa Shiozaki, Rajinder Shiwach, George Simpson, Joachim Springub, Klaus Steinwachs, Jaroslaw Strzelec, Louise Thurman, Gabor Vincze, David Walling, Ryszard Wardenski, Kauser Yakhin, Reiji Yoshimura, and Marcel Zins-Ritter.
Notes
Conflicts of Interest: YH has served as a clinical trial advisor for Otsuka Pharmaceutical Co., Ltd. and Chugai Pharmaceutical Co., Ltd.; has served on an advisory board for Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Eli Lilly Japan K.K., Chugai Pharmaceutical Co., Ltd., and Janssen Pharmaceutical K.K.; has received research grant from Otsuka Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Yoshitomiyakuhin Corporation, Janssen Pharmaceutical K.K., Shionogi & Co., Ltd., GlaxoSmithKline K.K., Pfizer Japan Inc., Sanofi K.K., Astellas Pharma Inc., and Sumitomo Dainippon Pharma Co., Ltd.; has received a lecture honorarium from Otsuka Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., GlaxoSmithKline K.K., Astellas Pharma Inc., MSD K.K., Asahi Kasei Pharma Corporation, Ono Pharmaceutical Co., Ltd., Yoshitomiyakuhin Corporation, and Eisai Co., Ltd.
TH has served on an advisory board for and/or received a lecture honorarium from Asahi Kasei Pharma Corporation, Astellas Pharma Inc., AbbVie GK, MSD K.K., Otsuka Pharmaceutical Co., Ltd., GlaxoSmithKline K.K., Shionogi & Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Pfizer Japan Inc., Meiji Seika Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Kyowa Hakko Kirin Co., Ltd., Kyowa Pharmaceutical Industry Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Yoshitomiyakuhin Corporation. SS, NS, and MN are employees of Chugai Pharmaceutical Co., Ltd.