Imaging Improves Diagnosis of Dementia with Lewy Bodies
Article information
Abstract
Dementia with Lewy bodies (DLB) is the second most common cause of degenerative dementia after Alzheimer's disease (AD), and is clinically characterized by the progressive cognitive decline with fluctuations in cognition and alertness, recurrent visual hallucinations and Parkinsonism. Once these characteristic symptoms of DLB emerge, discriminating it from AD is relatively easy. However, in the early disease stages, the clinical symptoms of various types of dementias largely overlap and it is difficult to distinguish DLB from other neurodegenerative dementias based on clinical manifestations alone. To increase the accuracy of antemortem diagnosis of DLB, the latest diagnostic criteria incorporate findings from 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy, or from neuroimaging such as computed tomography (CT), magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET). In the present guidelines, decreased dopamine transporter uptake revealed by SPECT or PET receives the greatest importance among various neuroimaging findings and is listed as one of the suggestive features. Supportive features that commonly present but are not proven to have diagnostic specificity include relatively-preserved medial-temporal-lobe structures, occipital hypoperfusion, and abnormal MIBG myocardial scintigraphy. In this paper, we review the major findings on various neuroimaging modalities and discuss the clinical usefulness of them for the diagnosis of DLB. Although there is not enough evidence to reach the conclusion, considering the accessibility in clinical practice, in our personal views, we recommend the use of brain-perfusion SPECT and MIBG myocardial scintigraphy to improve the diagnosis of DLB.
Historical Background of Dementia with Lewy Bodies
The concept of dementia with Lewy bodies (DLB) was first proposed in a case report by a Japanese psychiatrist, Dr. Kenji Kosaka, in 1976.1 Two years later, based on their neuropathological findings from three autopsied cases, Kosaka2 reported the characteristic distribution of Lewy bodies in cerebral cortices in the subjects with the later so-called "diffuse Lewy body disease (DLBD)". After the accumulation of similar cases, the term "Lewy body disease" was introduced and used to represent a spectrum of diseases involving an array of cognitive dysfunctions and motor symptoms.3 The spectrum could be divided into three types of disease according to the distributional pattern of Lewy bodies: the diffuse type, the transitional type and the brain stem and diencephalon type.4 The brain stem type is equivalent to Parkinson disease (PD), and the diffuse type is later designated as DLBD. Furthermore, Kosaka's group reviewed all reported DLBD cases in Japan and classified them into two subtypes; the common form contained various degrees of Alzheimer's pathology w hile the pure form never had such neuropathological findings. Then, in 1984, the term DLBD was proposed as a broader clinical entity which included various types of medical conditions with Lewy bodies.4 In short, Lewy body disease was referred to by various names such as Lewy Body disease, Lewy Body dementia, diffuse Lewy Body disease and DLB. The mixture of these words confused the clinicians till they reached a certain degree of consensus. Based on the accumulating cases, the definition of the term "DLB" was proposed and approved at the first international workshop in 1995.5 The publication of clinical guidelines for DLB highlighted a previously under-diagnosed condition and illuminated this clinical entity.6 However, several clinical studies revealed that the initial diagnostic criteria had insufficient sensitivity for reliable DLB recognition.7 To achieve acceptably-high sensitivity and specificity, the guidelines were revised and released anew in 2005.8 The revised version of guidelines has been well accepted and used worldwide both for clinical and research purposes.
Neuroimaging Studies in Dementia with Lewy Bodies
Currently, DLB is reported to be the second most common cause of degenerative dementia after Alzheimer's disease (AD).7-11 Since DLB causes characteristic impairments and disabilities such as neuroleptic hypersensitivity which may increase mortality, its prompt and correct diagnosis is very important. However, because the clinical symptoms of DLB and AD overlap in the early disease stages, it is difficult to distinguish DLB from AD based on clinical manifestations alone.
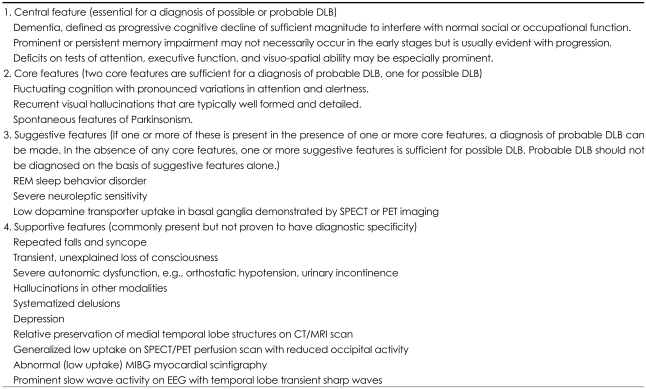
To increase the accuracy of antemortem diagnosis of DLB, the latest diagnostic criteria (Table 1) incorporate findings from 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy, or from neuroimaging such as computed tomography (CT), magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET).8 This imaging provides highly objective and precise data that contributes to the clinical diagnosis of DLB. For example, among three suggestive features in the diagnostic criteria of DLB: rapid eye movement (REM) sleep behavior disorder, severe neuroleptic sensitivity and low dopamine transporter uptake, the measurement of dopamine transporter activity in striatum by SPECT or PET enables the most objective and highly accurate assessment of characteristic abnormalities in DLB and could be a helpful diagnostic aid in discriminating DLB and AD. Supportive features include relatively preserved medial temporal lobe structures, occipital hypoperfusion/metabolism, and decreased MIBG uptake on myocardial scintigraphy. The results of CT, MRI, PET and brain-perfusion SPECT could be evaluated by statistical analyzing programs objectively.
In this paper, we give a brief overview of studies in DLB using various imaging modalities.
Morphological neuroimaging studies
Morphological neuroimaging, such as brain CT and MRI, is often performed for the clinical diagnosis of various types of dementia.12 The main aims of this imaging are the detection of cerebrovascular diseases, space-occupying legions such as hematoma and brain tumor, and the evaluation of the degree of cerebral atrophy.13-15 In regard to the diagnosis of DLB, there is no confirmed characteristics with a significant diagnostic value from morphological neuroimaging.12 In the consensus guidelines,8 relatively-preserved medial-temporal-lobe structures on CT/MRI are alone listed as one of the supportive features in DLB. These are commonly present but are not proven to have diagnostic specificity.
Region-of-Interest Based Analyses
Previous morphological neuroimaging studies used region-of-interest (ROI) based analyses to assess the degree of cerebral atrophy. In this method, one needed to place the ROIs manually, and only selected regions could be investigated.
Morphological neuroimaging studies using visual inspection or ROI techniques demonstrated that the extent of medial temporal lobe atrophy was severer in AD compared to DLB.16-20 Hashimoto et al.17 reported that hippocampal volume in DLB was significantly larger than that in AD, but significantly smaller than in the normal control. In their study, no significant differences were found in the amygdala and whole-brain volume between DLB and AD, though the amygdala and whole brain were smaller in DLB compared to the control. These findings were consistent with the results of the study by Barber et al.16 that demonstrated that the subjects with DLB had significantly larger temporal-lobe, hippocampal, and amygdala volumes than those with AD. Their results also reported that significant ventricular dilation was observed in the subjects with AD, vascular dementia (VaD) and DLB, and only the subjects with DLB had a relatively preserved volume of the cerebrum. The medial temporal lobe atrophy in DLB has been reported to be related to age and cognitive impairment.19,20 Therefore, clinicians need to take the patients' history carefully and examine clinical symptoms thoroughly before interpreting the results of neuroimaging.
Besides the temporal lobe, other cerebral structures have been studied morphologically. Almeida et al.21 reported that there were no significant differences in caudate nucleus volume in DLB, AD, PD, PD with dementia (PDD) and controls. However, Cousins's group demonstrated that the volume of putamen was smaller in DLB compared to AD and normal controls.22 Regarding the atrophy of substantia innominata, Hanyu and his colleagues23 showed significant reduction of the volume only in DLB, while the volume was preserved in AD, VaD and the control.
Voxel-Based Morphometry
Voxel-based morphometry (VBM) is one of the neuroimaging analysis techniques that investigate morphological differences by using statistical parametric mapping (SPM).24,25 VBM enables objective and quantitative analysis of brain structure.
Compared to the normal control, decreased volume of the temporal and frontal lobes and insular cortex was found in DLB by Burton's group.26 The MRI study done by Whitwell et al.27 revealed decreased volume of the dorsal midbrain, substantia innominata and hypothalamus. Brenneis et al.28 demonstrated the atrophy of lateral prefrontal cortex and left premotor cortex in DLB.
In regard to the comparison between DLB and AD, AD patients showed decreased volume in the medial temporal lobe, hippocampus, amygdala and thalamus in Burton's study,26 in the hippocampus and temporo-parietal cortex in Whitwell's study,27 and in the temporal and frontal lobe in Beyer's study.29
Taken together, we can conclude that the relatively-preserved volume of the medial temporal lobe could be a support for the diagnosis of DLB, especially for the discrimination of DLB from AD.
Diffusion Tensor Magnetic Resonance Imaging
Diffusion tensor imaging (DTI) is an advanced neuroimaging technique that performs a more sensitive investigation of tissue microstructure compared to conventional imaging methods, and enables the extraction of white matter connections of the brain using tractography.30-33 A number of studies have reported the usefulness of DTI for investigating the pathophysiological mechanisms of DLB.34,35 Firbank et al.36,37 demonstrated a decreased fractional anisotropy in the parietal lobe in DLB and an increased apparent diffusion coefficient in the left temporal lobe in AD, suggesting the region-specific disruption of the connectivity in respective dementias. Bozzali et al.38 revealed abnormalities in brain regions with long connecting tracts, which suggested the involvement of association cortices in neurodegenerative process in DLB. This group also demonstrated that no significant abnormality was found in the occipital region of AD subjects and normal controls, and suggested that DTI could be a support tool for the diagnosis of DLB.39 Ota and his colleagues40 applied DTI to examine the mechanisms of visual hallucination in DLB and indicated the possible involvement of inferior longitudinal fasciculus which played an important role in visuo-spatial cognition.
Functional neuroimaging studies
The initial consensus guidelines for the clinical diagnosis of DLB5 proved to be insufficiently sensitive for reliable DLB recognition.7 To increase the accuracy of the clinical diagnosis of DLB, the latest diagnostic criteria incorporate findings from SPECT or PET, and include reduced occipital perfusion as a supportive feature.8
Single Photon Emission Computed Tomography
Brain-perfusion SPECT is often performed for the diagnosis of dementia, along with CT and/or MRI. Characteristic hypoperfusion in the hippocampus, posterior cingulate gyrus and temporoparietal cortex is very useful for the early detection of AD.41-44 The most important finding on brain-perfusion SPECT in DLB is occipital hypoperfusion. This hypoperfusion is listed as a supportive feature in the consensus guidelines.8
To increase the accuracy of the clinical evaluation and to achieve the highest possible agreement between different readers, algorithms for analyzing SPECT images have been developed and used in the clinical setting. Two widely used methods are SPM45,46 and three-dimensional stereotactic surface projection (3D-SSP).47-51 In both algorithms, SPECT images are anatomically standardized and statistically analyzed. Both SPM and 3D-SSP enable us to detect cerebral regions with lowered blood perfusion with statistical significance. Since SPM uses the t-test for statistical analysis, when the degrees of freedom of the sample are small, the sensitivity can be lowered while specificity remains high. 3D-SSP has high sensitivity and specificity. However, as the images are projected to the surface of the schematic brain, it is difficult to determine the exact region of abnormal blood flow. To make better use of the advantages discussed above, Matsuda et al. developed a new qualitative analysis method named eZIS.52-55 This method is accompanied by a database prepared from age-matched normal controls, and compares the results of individual subjects with the database for statistical analysis. The extent of the decrease of cerebral blood flow is expressed as a set of Z-values. Each value equals the division of the difference between the mean of the normal controls and the result from the subject by the standard deviation of the normal controls. The Z-value results are shown as a projection chart on a schematic brain. Furthermore, Takeuchi et al. developed a fully-automated rCBF quantification method, 3DSRT, which allowed objective assessment of rCBF by setting the ROIs identically on anatomically-standardized SPECT images.56-59 3DSRT includes anatomical standardization of images employing SPM which is used only for that purpose, rCBF quantification using a three-dimensional stereotactic ROI template, calculation of CBF and display of the results.
The initial SPECT studies using 99mTc-hexamethyl propyleneamine oxime (HMPAO) as a tracer suggested limited value of such studies for the differential diagnosis of DLB from AD.60-62 However, the following research demonstrated that occipital hypoperfusion revealed by brain-perfusion SPECT had high enough sensitivity and specificity to distinguish DLB from other dementias.
Ishii et al.63 performed HMPAO-SPECT in DLB, AD and healthy control, and analyzed their results by using SPM. The analysis results revealed decreased cerebral blood flow in the occipital lobes. Lobotesis et al.64 reported that blood perfusion in AD and DLB differed only in occipital areas and distinguished DLB from AD and control subjects with a 65% sensitivity and 87% specificity which were equal to Varma's study.65 Colloby and his colleagues66 investigated the results of the HMPAO-SPECT performed in 48 AD, 23 DLB and 20 control subjects, and indicated hypoperfusion in both parietal and occipital regions of the brain in DLB compared to the AD group.
Pasquier et al.67 used 99mTc-ethylcysteinate dimer (ECD) as a tracer for their brain-perfusion-SPECT study and obtained a similar sensitivity of 65% for the diagnosis of DLB. Our group performed ECD-SPECT in 25 DLB subjects and analyzed the results by using eZIS.68 The sensitivity calculated in our study was 68% and this value was equivalent to the previous studies. On the whole, the sensitivity of brain-perfusion SPECT has been reported to be around 60-70%.
We performed quantitative analysis of brain-perfusion SPECT in DLB and demonstrated the clinical usefulness of an automated cerebral blood flow quantification program, 3DSRT, for the diagnosis of DLB.69 3DSRT enabled us to estimate the blood perfusion in the region of interest. In the comparison of the usefulness of different SPECT analyzing programs, the 3DSRT analysis of brain-perfusion SPECT in DLB showed higher sensitivity (76%) compared to that of eZIS (68%).68
Positron Emission Tomography
Cerebral metabolism is often measured for the clinical diagnosis of dementias by using fluorodeoxyglucose (FDG) and PET.70 Characteristic regional hypometabolism could be useful to differentiate DLB from other dementias.
Albin and his colleagues71 were the first to report hypometabolism in the occipital association cortex and primary visual cortex in DLB. In the following year, Imamura and his colleagues72 verified Albin's findings by investigating a bigger sample. Ishii et al.73 performed FDG-PET and investigated the regional cerebral metabolic rate of glucose in three groups: DLB, AD and controls. Their results demonstrated that the decreased metabolic rate in the occipital region could distinguish DLB from AD with a sensitivity and specificity of 92% each. His group suggested that occipital hypometabolism might be associated with visual hallucinations in DLB.74 The PET study conducted by Higuchi's group indicated that the glucose metabolism was significantly reduced in the visual association cortex of DLB subjects compared to the AD group.75 Minoshima et al.76 examined cerebral glucose metabolism in the subjects with autopsy-confirmed DLB (n=11) and AD (n=10) along with 53 probable AD subjects including 13 cases who later received a clinical diagnosis of DLB. In their study, hypometabolism in the primary visual cortex distinguished DLB from AD with 90% sensitivity and 80% specificity.
Dopamine Transporter Imaging
In the latest criteria for the clinical diagnosis of DLB, decreased dopamine transporter (DAT) uptake in the basal ganglia is listed as one of the suggestive features.8 If one or more suggestive features is present in combination with one or more core features, a diagnosis of probable DLB can be made, whereas in the absence of any core features, one or more suggestive features is defined as possible DLB. Thus, DAT imaging receives the highest priority among various neuroimagings. To investigate the striatal DAT function in DLB, two kinds of ligands for SPECT imaging are used: [123I]-2beta-carbometoxy-3beta-(4-iodophenyl)-N-(3-fluoropropyl) nortropane (FP-CIT) and [123I]-2beta-carbomethoxy-3beta-(4-iodophenyl) tropane (beta-CIT). Donnemiller et al.62 performed beta-CIT SPECT on 7 probable DLB, 6 probable AD and 3 normal controls, and indicated that low DAT function was useful to discriminate DLB from AD and normal controls. His group also performed SPECT imaging by using beta-CIT on 20 DLB patients, 24 subjects with PD and 10 normal controls, and demonstrated that DAT imaging might be useful to distinguish DLB and PD.77 As to FP-CIT, O'Brien's group used this ligand and performed DAT imaging on probable/possible DLB, PD, PDD, AD and normal controls.78 Their results demonstrated that the sensitivity, specificity and positive predictive value for the differentiation of DLB from AD were 78%, 94% and 90%, respectively, though it was difficult to separate DLB from PD with and without dementia. McKeith et al. lead a multicenter study about FP-CIT SPECT on DLB and assessed 326 patients including probable (n=94) or possible (n=57) DLB or 147 non-DLB dementia.79 They calculated a mean sensitivity of 77.7% for discriminating probable DLB and specificity of 90.4% for excluding non-DLB dementia. Based on these findings, low DAT uptake was given the diagnostic importance as a suggestive feature of DLB.
Despite these clinical studies, the usefulness of DAT imaging for the diagnosis of DLB remains controversial.80 Further studies are awaited.
123I-Metaiodobenzylguanidine
MIBG is a physiological analogue of norepinephrine and competes with it for neuronal uptake at the sympathetic nerve terminal.81-83 MIBG myocardial scintigraphy was originally used to assess myocardial sympathetic nerve damage in heart disease.84-86 Later this method was applied to detect cardiac sympathetic denervation in PD and clinically used to discriminate PD from other neurological disorders with extrapyramidal signs (EPS).87-89 The heart-to-mediastinum ratio of myocardial MIBG uptake and the washout rate in percent are used to assess the severity of the postganglionic cardiac sympathetic nerve denervation. Recent studies have demonstrated that MIBG myocardial scintigraphy is useful for the clinical diagnosis of DLB.90-93 The latest guidelines include abnormal MIBG myocardial scintigraphy as one of the supportive features which commonly present in DLB but are not proven to have diagnostic specificity.
Yoshita et al.90 first reported the clinical usefulness of MIBG scintigraphy to distinguish DLB from other dementias by investigating 14 subjects with DLB. The clinical difficulty of discriminating DLB from AD stems from the fact that there are many AD cases with EPS and DLB cases without Parkinsonism. MIBG myocardial scintigraphy detected disturbances of cardiac sympathetic nerves in patients with DLB regardless of clinically-evident Parkinsonism, whereas AD patients with EPS showed no significant decrease of MIBG uptake.94,95 Yoshita's group performed MIBG myocardial scintigraphy on 37 probable DLB patients including 7 cases without EPS, 42 probable AD subjects and 10 normal controls.95 They reported that setting the cut-off value of the heart-to-mediastinum ratio at 1.68 yielded 100% sensitivity and specificity for differentiating DLB from AD. Similarly, setting the washout-rate cut-off value at 23.6% yielded 87% sensitivity and 83% specificity.
In comparing the clinical value of brain-perfusion SPECT and MIBG myocardial scintigraphy, Hanyu et al.96 observed decreased heart-to-mediastinum ratios in all 19 DLB patients, while only 14 of them showed occipital hypoperfusion. They concluded that MIBG myocardial scintigraphy could improve sensitivity in the detection of DLB. The results of our study were consistent with those from Hanyu's study. Twenty-four of 25 subjects (96%) had decreased cardiac MIBG uptake in the delayed image (3 hours after injection), while occipital hypoperfusion was observed in only 68% and 76% of them by applying, respectively, eZIS and 3DSRT.68 Most studies on MIBG myocardial scintigraphy thus far have been conducted by Japanese groups. However, a Spanish team recently reported that a heart-to-mediastinum-ratio cut-off value of 1.36 differentiated DLB from other dementias with a sensitivity of 94% and a specificity of 96%.97
At the 4th International Workshop on DLB and PDD, there was a heated debate about whether abnormal MIBG myocardial scintigraphy should be included as a suggestive feature for DLB. At present, there is not enough evidence to thoroughly validate the usefulness of MIBG myocardial scintigraphy in the diagnosis of DLB, and we still need to accumulate more data.
Conclusion
DLB has been reported to be the second most common type of degenerative dementia. However, DLB is often under-diagnosed and its discrimination from other dementias is very difficult, especially at its early stages. Since DLB has specific impairments and functional disabilities, its early and accurate diagnosis is very important for a better prognosis. To achieve such an improved prognosis, neuroimaging studies and MIBG myocardial scintigraphy play important roles. Further research is needed to establish their clinical usefulness even further.
Acknowledgments
The neuroimaging studies by the authors' group were supported by a Grant-in-Aid for Young Scientists (B)(MT: 20790852) from the Japan Society for the Promotion of Science (JSPS).