The Role of BDNF as a Mediator of Neuroplasticity in Bipolar Disorder
Article information
Abstract
The cognitive impairment and neuroanatomical changes that takes place among patients with bipolar disorder (BD) patients has been well described. Recent data suggest that changes in neuroplasticity, cell resilience and connectivity are the main neuropathological findings in BD. Data from differential lines of research converges to the brain-derived neurotrophic factor (BDNF) as an important contributor to the neuroplasticity changes described among BD patients. BDNF serum levels have been shown to be decreased in depressive and manic episodes, returning to normal levels in euthymia. BDNF has also been shown to decrease as the disorder progresses. Moreover, factors that negatively influence the course of BD, such as life stress and trauma have been shown to be associated with a decrease in BDNF serum levels. These findings suggest that BDNF plays a central role in the progression of BD. The present review discusses the role of BDNF as a mediator of the neuroplastic changes that occur in portion with mood episodes and the potential use of serum BDNF as a biomarker in BD.
INTRODUCTION
Bipolar disorder (BD) is a highly disabling chronic mood disorder characterized by the presence of manic and depressive symptoms and a lifetime prevalence of 3.9%.1,2 Epidemiological studies indicate a role for both biological and environmental factors in the ethiopathogenesis of BD. Due to the high heritability and familial relative risk reported in BD, there is little doubt that molecular genetics play an important role. However, the genetic basis for this illness remains elusive.3,4 An emerging body of evidence suggests that environmental stressors may trigger mood episodes.5 Indeed, it is known that stressors are more likely to be involved in the precipitation of the first episodes, but less so with subsequent episodes.6,7 In the same vein, cognitive impairment has been also demonstrated in bipolar patients with a history of multiple mood episodes.8 In terms of neuropathological findings, data suggest that changes in neuronal plasticity, particularly in cell resilience and connectivity, are the main finding in BD.9
The brain-derived neurotrophic factor (BDNF) plays an important role in a variety of neural processes during the development of both animals and humans. Initially, BDNF is important for neurogenesis, neuronal survival, and normal maturation of neural development pathways. In the adult, BDNF is not only important for synaptic plasticity and dendritic growth, but also for long-tem memory consolidation.10
In the present review, we describe the recent findings in the gene expression and mechanisms of action of BDNF as well as how psychosocial stress and BD mood episodes modulate BDNF brain levels. We also discuss the proposal of BDNF as a potential biomarker in BD.
BDNF GENE EXPRESSION AND MECHANISM OF ACTION
BDNF is a member of the growth factor family, which is involved in promoting synaptic efficacy, neuronal connectivity and neuroplasticity.6 It has emerged as a key mediator of synaptic plasticity, neuronal connectivity and dendritic arborization.11,12 Together with other biological factors, such as neurotransmitters, hormones and other neurotrophins, BDNF orchestrates mechanisms of neuronal plasticity and survival.
Transcription
BDNF has an extremely complex genomic structure. The human gene presents eleven exons and nine functional promoters, producing up to seventeen different transcripts which encode for the same protein.14 In the rat, for instance, Bdnf gene has nine exons with its own promoter, producing nine different transcripts.15 Such a complex set of genomic promoters is thought to mediate accurate control of BDNF production. Cumulative evidence indicates that these transcripts are differentially distributed across brain regions in different cell types and even within different parts of the neuron. For example, in the rat, exon III transcripts are detected only in cell bodies, whereas exon IV transcripts are found in cell bodies and dendritic processes of visual cortex neurons.16 These promoters are differentially activated in response to diverse and varied signaling events, including epigenetic regulation. Recent reports have suggested a pathophysiological role for BDNF in major depression and suicide.17 Kim et al. 2010 have suggested that the BDNF messenger RNA (mRNA) expression is reduced in peripheral blood mononuclear cells of patients with major depression. This alteration of BDNF mRNA expression was more pronounced in recent suicide attempters.
There is evidence showing that chromatin remodeling involving the BDNF gene may be associated with the deleterious effects of stress and with antidepressant response. More specifically, Tsankova et al.18 found that chronic defeat stress, a mouse model of depression, induced a 3-fold downregulation of Bdnf mRNA expression in the hippocampus, an effect that was mediated by repressive histone methylation and consequent decrease in the expression of Bdnf transcripts III and IV. Moreover, chronic treatment with imipramine increased histone acetylation at these same promoters, thereby normalizing the expression of Bdnf transcripts III and IV and total protein. More recently, Yasuda et al.19 showed that the mood stabilizers lithium and valproate increased Bdnf transcript III in rat cortical neuronal cultured cells. Together, these studies strongly suggest that the regulation of BDNF transcription may be a key target for the effects of antidepressants and mood stabilizers.
Translational and post-translational modifications
BDNF transcripts are translated into proBDNF, which binds to sortilin in the Golgi to facilistate its appropriate folding, trafficking and secretion (Figure 1).20,21
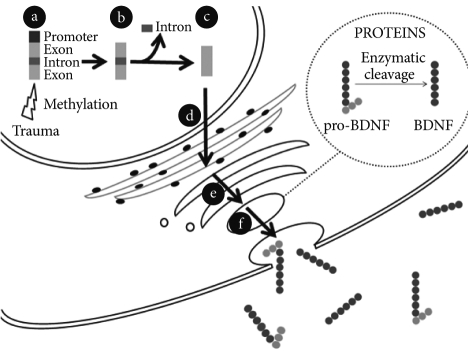
BDNF synthesis and release from neurons. a: BDNF gene: promoters, exons and introns. The BDNF gene expression may be modulated by epigenetic mechanisms. Trauma can induce methylation of the promoters of the BDNF gene and therefore inhibit their transcription. b: Different mRNA transcripts can be produced depending on which of the promoters is activated. c: An alternative splicing mechanism removes the introns out and leads to the formation of a processed mRNA molecule ready to be translated. d: The mRNA molecule translocates out of the nucleus into the cytoplasm and is translated into proBDNF in the endoplasmic reticulum. e: The newly synthesized proBDNF heads to the Golgi apparatus and is then cleaved into mature BDNF by endoproteases. f: BDNF-containing vesicles merge to the cell membrane in a Ca2+-dependent way and release BDNF to the extracellular space.
It has been demonstrated that a single nucleotide polymorphism in the BDNF gene, substitution of a valine for a methionine at the codon 66 (val66met), is involved in altered trafficking of BDNF. Such change seem to take place due to a reduced interaction of BDNF and sortilin inducing metBDNF aggregation to the cell body of neurons and thus preventing it to interact with synaptophysin. That would in turn reduce the BDNF secretion into the synapse.20 Further, knock-in BDNFmet/met mice have abnormal dendritic arborization in the dentate gyrus and display anxious-related behaviors that are not normalized by antidepressant treatment.22 In BD patients, the val66met substitution in the BDNF gene has been associated with impaired cognitive performance,23 and suicidal behavior.24 It has also been reported a differential response to lithium prophylaxis25 and decreased prefrontal cortical volume among patients with BP who presented the val66met substitution in the BDNF gene.26 In addition, val66val genotype showed an association with increased risk of rapid cycling27,28 and childhood onset of BD.29,30 Serum levels of BDNF have also been evaluated in euthymic patients with both val/val and met carriers as compared to controls.31 The val66met was not associated with a differential serum level in BD patients.
The BDNF secretion can be either constitutive or, more frequently, regulated by stimuli.32 This activity-dependent secretion, a feature characteristic of BDNF and not of any other neurotrophin or growth factor,33 may be an important factor in mood regulation. Along with slow effects that require protein synthesis, BDNF exerts rapid signaling events that regulate synaptic plasticity.34 For example, inducing phosphorylation of synapsin and thereby increasing glutamate and GABA release.35 BDNF can also increase ion influx through N-methyl-D-aspartate receptors and then synaptic strength.36 Thus, BDNF is able to regulate synaptic plasticity and recent findings suggest that mood disorders would be associated with alterations in information processing within neural networks.37 A large proportion of neuronal BDNF is secreted in the pro-form (proBDNF) which is subsequently converted to the mature form (mBDNF) by endoproteolytic cleavage.38 Lee et al.39 suggested that the extracellular conversion from premature into mature forms was achieved through serine protease plasmin and by selective matrix metalloproteinases. The study of the conversion of proBDNF into BDNF is a matter of importance since these structures elicit differential biological effects. For instance, proBDNF preferentially binds to pan-neurotrophin receptor p75NTR related to apoptosis while mature BDNF acts at tyrosine kinase (Trk) type-B receptor. On the contrary to p75NTR, the BDNF binding to the Trk B receptor initiates intracellular cascades involved with cellular survival, growth and differentiation via mitogenactivated protein kinase, phosphatidylinositol 3-kinase, and phospholipase C-g signal transduction pathways.37 They can induce dendritic sprouting by means of cytoskeleton modulation40 (Figure 2). These findings have led to the "ying-yang hypothesis" where pro- and mature neurotrophins draw out opposite biological actions by means of differential receptors.21 The mature BDNF is critical for long-term potentiation, whereas proBDNF facilitates long-term depression.41 Depending on the localization, these molecules may display opposite effects. Intrahippocampal infusion of BDNF produces antidepressant effects, whereas it may present a pro-depressive role when the infusion is carried out in the ventral tegmental area/nucleus accumbens reward system.42
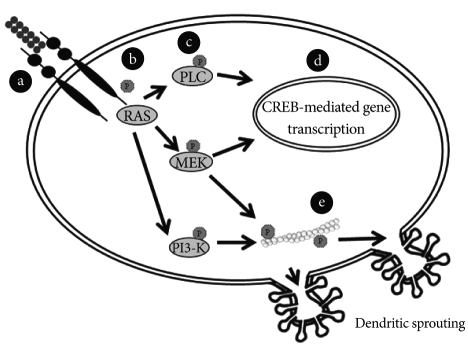
BDNF-activated transduction pathways induce dendritic sprouting. a: BDNF binds to tyrosine kinase receptor type-B and induces the dimerization of the receptor. b: Binding of BDNF induces TrkB autophosphorylation at specific tyrosine residues of the receptor and thus creates binding sites for specific proteins. c: Three main intracellular signalling cascades are activated by TrkB: Ras-mitogen-activated protein kinase (MAPK) pathway, the phosphatidylinositol 3-kinase (PI3K)-Akt pathway and the PLCg-Ca2+ pathway. d: Activation of PLC-g leads to the release of calcium from the endoplasmic reticulum and to activation of a calcium-calmodulin-dependent kinase II (CAMKII), ending in phosphorylation of CREB and activation of transcription. Activation of the MAPK pathway can also regulate transcription through phosphorylation of CREB. e: Signaling pathways mediate BDNF-promoted modifications of dendritic morphology. Simultaneous triggering of the PI3K and MAPK pathways concurrently alters both actin and microtubule dynamics and changes downstream dendrite branching.
PSYCHOSOCIAL STRESS, BIPOLAR DISORDER AND BDNF
BDNF expression has been shown to be regulated by stress responsive corticosteroids.43 The interaction between BDNF and corticosteroids appears to play a key role in the environmentally-mediated vulnerability to psychopathology.44 Early exposure to traumatic life events and posttraumatic stress disorder, as well as depression, has been associated with hypothalamic-pituitary adrenal (HPA) axis dysfunction and enduring stress response alterations.45 In fact, Schule and colleagues46 showed that patients with BDNF met/met polymorphism had higher HPA axis activity during dexametasone/CRH test. Glucocorticoids and mediators of stress interact with neurotransmitter systems resulting in neuroplastic alterations seen in hippocampus, amygdala and prefrontal cortex.47 For instance, chronic stress in animal models is related to abnormal neuronal remodeling in the prefrontal cortex,48-50 particularly in glial cells,51 and amygdala.52
Accordingly, decreased BDNF levels have been associated with decreased hippocampal volume. Studies have reported smaller hippocampal volumes in patients with early life stress and child sexual abuse. Depressed women with a history of child abuse have an 18% smaller left hippocampal volume than non-abused women.53 Remarkably, these apparent differences in hippocampal size may be reversible with antidepressant treatment, consistent with a function of neurotrophic factors in neural plasticity in the hippocampus.54 Taken together, these data suggest that BDNF-related neuronal plasticity may be an important mediator of the effects of psychosocial stress on psychopathology.
Stress and bipolar disorder
Chronic stress is known to induce hyperactivation of amygdala, enhancing amygdala-dependent unlearned fear, fear conditioning, and aggression.55 Similarly, many of the symptoms experienced by patients with BD appear to be associated with abnormalities in emotional processing which involve amygdale-related circuitry. In this same vein, an enlargement of the amygdala has been described as the most prominent abnormality in BD.56-59 In addition to structural changes in this circuitry, functional neuroimaging studies indicate increased activity in the amygdala during acute mood episodes60,61 and impairment in amygdala-dependent tasks, such as facial recognition tasks.62 Emotional memory has also been evaluated as an amygdala-related cognitive task. Contrary to controls, patients with BP had no enhancement of memory for the emotional content of the story and the subjective perception of the emotional impact of the emotional condition was significantly different from that of the neutral condition in controls but not in people with BD.63 These findings suggest that amygdala and its related circuits seem to be overactive and dysfunctional in patients with BD. It may be possible that the gate system to code experiences as stressful is overactive and defective in BD patients.64 Such malfunctioning would render bipolar patients more vulnerable to stress. It has been reported that childhood trauma associated to BD may lead to more complex psychopathological manifestations.65,66
It is noteworthy that the same genes associated with BD have been also been implicated in decreased resilience to stress. BDNF and other neurotrophic factors are believed to counteract the negative impact of stress hormones on the hippocampal volume.67 Together, BDNF and corticosteroids may play a role in the environmentally mediated vulnerability to cognitive impairment in BD.68 In a BD sample, those with history of traumatic experiences had lower serum BDNF which may be related to an incremented load of stress.69
BDNF AS A BIOMARKER IN BIPOLAR DISORDER
Diagnostic criteria, as well as other aspects of clinical management such as treatment monitoring, are still essentially based on clinical symptomatology. There is a clear need for biological markers as complements for diagnostic and prognostic assessments in order to improve our management of BD. Recent evidence suggests that BDNF might be a potential marker.85
BDNF as a biomarker of neuronal dysfunction
BDNF is highly expressed in the cerebral cortex and hippocampus, brain areas that are known to regulate complex brain functions such as memory and emotion. It has been demonstrated that BDNF plays a key role in long-term potentiation, one of the most accepted models of learning and memory. For instance, the administration of exogenous BDNF to genetically modified mice deficient in BDNF or its receptor TrkB rescue the impairment in LTP process.70 In addition, transgenic mice lacking BDNF or TrkB demonstrate poorer performance than their wild-type littermates in the Morris water maze, a hippocampal-dependent spatial learning task.71 These and other studies suggest that abnormalities in the BDNF-signaling system might be implicated in the cognitive decline observed in certain neuropsychiatric disorders, such as BD,72 major depression73 and schizophrenia.74
There is an emerging body of evidence indicating that BDNF is associated with the mechanism of action of antidepressants and mood stabilizers.75,76 In the cerebral cortex and hippocampus, it has been reported increased BDNF expression after chronic antidepressant treatment.73 Moreover, it was demonstrated that the blockage of BDNF-signaling with either a tyrosine receptor kinase inhibitor or a mitogen-activated extracellular regulated kinase (ERK) kinase/ERK inhibitor attenuated the antidepressant effects of BDNF.77 In this same vein, the chronic administration of lithium and valproate increased BDNF content in the rat hippocampus and prefrontal cortex.78,79 In addition, the depressive behavior induced by social defeat stress in rats is prevented by blockage of BDNF in the ventral tegmental area.42
Taken together, these observations suggest that decreased BDNF may be a marker of neuronal dysfunction, possibly mediating cognitive impairment, which can be reversed by proper treatment. Therefore, a deeper understanding about the molecular determinants involved in BDNF-signaling cascades may provide a means for monitoring treatment response and disease progression as well as the development of novel agents for the treatment of BD.
BDNF and mood episodes
It has repeatedly been described that cognitive dysfunction in patients with BD is not only present during mania and depression but also in euthymia.8,80-82 Such cognitive impairment has been construed as a consequence of the cellular strain imposed by recurrent mood episodes.83-85 In this sense, the burden of repeated mood episodes would translate into episode recurrence, cognitive impairment, disability and premature death.83,86
Some growth factors, including BDNF, which are altered by stress, have been shown to be modified in BD.10 It has been demonstrated that serum BDNF levels decrease during manic and depressive episodes in both treated and drug-free subjects when compared to normal controls and to unipolar depression and that BDNF levels are negatively correlated with severity of manic and depressive symptoms.87-90 Moreover, Tramontina et al.91 showed that BDNF levels of manic patients were lower than those of healthy controls and that the significant difference vanished after successful treatment. In the light of such data, the decrease of BDNF levels may be conceived as a statedependent biomarker of BD as reported in the meta-analysis of Lin.92 It has also been described that BDNF levels are decreased in chronic or late stage individuals with BD compared to early stages of the illness.93 Moreover, accelerated age-related decreased of BDNF was described.94 In addition, serum neurotrophin-3,95 neurotrophin 4/5 and glial cell line-derived neurotrophic factor levels96 were showed to be increased during acute mood episodes.95-97 Taken together, these findings suggest an orchestrated change in the pattern of neurotrophin expression during mood episodes. As the disorder progresses, this pattern seems to be altered even between episodes, suggesting a trait characteristic of later stages of illness, characterized by chronic subsyndromic symptomatology, cognitive impairment and functional decline.44
Changes in neurotrophins seem to occur in portion with changes in other biomarkers such as oxidative stress markers and molecules related to inflammation.98 Taking this into account, we have postulated that such systemic changes related to mood episodes would be better measured by a composite assessment of peripheral toxicity. Thus we have recent put forward the notion that a systemic toxicity index would be a useful construct as a means to assess peripheral changes in mood episodes.99
CONCLUSION
Recent evidence suggests that BDNF might be a potential state marker is BD. The fact that serum BDNF levels are decreased during manic and depressive episodes, strongly suggest that the normalization of BDNF levels may be associated with clinical stabilization.87-90 However, these assumptions are based on case-control studies. Few longitudinal studies have been developed up to the present day.91,104 Another limitation of this hypothesis is that it is based fundamentally in studies conducted with animal models or human peripheral blood, and the presumption that such findings might be occurring in the human brain needs to be confirmed. In this regard, a postmortem study showing that individuals under antidepressant medications at the time of death had higher hippocampal BDNF expression than individuals not on antidepressants105 further support the role of BDNF in the treatment of mood disorders. The development of specific ligands for TrkB receptors could be extremely valuable in future positron emission tomography studies in humans.
At the same time, it is not appropriate to rely only in episodic alterations to fully explain the pathophysiology of BD. Impairments in neuronal plasticity and resilience could be the neuropathological hallmark of BD, corresponding to more enduring changes in the brain of patients. Morphometric studies have demonstrated that patients with BD have enlargement of third and lateral ventricles and reduced gray matter volumes of orbital and medial prefrontal cortices, ventral striatum and mesotemporal cortex, as well as an increase of the size of amygdale.7 Notably, it was reported that such neuroanatomical changes tend to be more pronounced with repeated episodes.100 Apart from neuroanatomical changes, impairment in cognitive function has been also demonstrated in manic, depressed as well as euthymic bipolar patients.80,81,101,102 Such impairment seems to be related to indicators of the severity of illness, such as the presence of psychotic symptoms, longer duration of illness and higher number of manic episodes.103
Several transversal and longitudinal brain imaging studies demonstrated that lithium treatment increases cerebral cortical gray matter content and hippocampal volume in patients with BD.106-111 It can be presumed that these findings may be related to neurotrophic effects of lithium, especially by increasing cerebral BDNF.108,109 This hypothesis is largely supported by studies in rodents showing that lithium, valproate and antidepressants increase BDNF levels in the hippocampus and prefrontal cortex, brain regions known to be involved with mood regulation.78,79 Thus, we believe that substances that are able to increase cerebral BDNF expression have the potential to affect human affective responses and exert mood stabilizing effects, and that this rationale should be included in the investigation of new treatment approaches. In this regard, recent new promising drugs in the field of BD such as protein kinase C inhibitors112 and glutamate modulators113 may regulate the expression of BDNF through downstream effects on transcriptional factors and gene expression.
Finally, genetics is another promising field of research with a potential to unravel individual differences in treatment response related to distinct genetic predisposition. For instance, while a recent study showed that the BDNF val66met polymorphism is not associated with antidepressant-induced mania,114 another study found that individuals val/met for this polymorphism may be better responders to lithium prophylaxis.25 Obviously, longitudinal studies are necessary to better determine the role of the val66met polymorphism in treatment response. In addition, studies addressing the involvement of other single nucleotide polymorphisms of the BDNF gene, as well as the interaction between BDNF and other functional genes are warranted.