Epigenetic Regulation of BDNF Gene in Response to Stress
Article information
Abstract
Neuronal plasticity induced by changes in synaptic morphology and function is well known to play a pivotal role in leaning and memory as well as adaptation to stress. It is suggested that these plastic changes are due to orchestration of alterations in gene expression in the brain. Recent advances in molecular biology have provided evidence that epigenetic mechanisms, such as DNA methylation and histone modification, are crucial to gene transcription in the mammalian brain. Our research group has recently investigated the involvement of histone actylation at the promoter of the brain-derived neurotrophic factor (BDNF) gene in stress-induced reduction in BDNF, as well as in fear conditioning-induced enhancement of BDNF, in the rat hippocampus. The results of the stress study demonstrated that single-immobilization stress significantly reduced the levels of total, exon I, and exon IV BDNF mRNA, and also significantly reduced acetylation levels of histone H3, but not H4, at the promoter of exons I, IV, and VI. The results of the fear conditioning study showed that footshock stress significantly increased the levels of total, exon I, and exon IV BDNF mRNA, with significantly increased acetylation levels of both histone H3 and H4, at the promoter of exons I and IV, followed by enhanced freezing to fear-context exposure. These findings suggest that changes in BDNF transcription in the rat hippocampus in response to stressful stimuli are, at least in part, regulated by histone acetylation status.
Neuronal plasticity mediated by changes in synaptic morphology and function is well known to be involved in learning and memory as well as adaptation to stress. In addition, the regulation of gene expression in response to environmental stimuli is one of the major mechanisms of neural plasticity. With regard to gene expression, growing evidence suggests that epigenetic mechanisms, such as histone modifications and DNA methylation, play an important role in the alteration of gene expression in the brain. One type of histone modification, histone acetylation, regulates a global chromatin environment. While increased acetylation of histone (H3, H4) changes chromatin structure and subsequently leads to euchromatin status, where DNA is kept accessible for transcription, decreased acetylation of histone (H3, H4) leads to heterochromatin status, where chromatin is inaccessible for transcription. The collective evidence suggests that epigenetic regulation of gene expression is crucial for neuronal plasticity.
Levenson and Sweatt1 demonstrated that increased acetylation of histone H3 was required for long-term memory formation, and that histone deacetylase (HDAC) inhibitors, at least in part, contributed to the induction of long-term potentiation (LTP) and long-term memory. Similarly, Korzus and colleagues2 reported that decreased activity of histone acetyltransferase (HAT) disturbed the formation of long-term memory, and that the administration of HDAC inhibitors ameliorated this disturbance. Furthermore, several recent studies have demonstrated that chromatin modification by histone acetylation and DNA methylation is associated with initial memory formation and consolidation.3-5
Likewise, stress exposure is also reported to change gene transcription through epigenetic mechanisms. One form of early adverse experience, low maternal care, was shown to be associated with increased DNA methylation and decreased histone actylation (H3) at the promoter of exon I of the glucocorticoid receptor (GR), resulting in impaired hippocampal expression of GR in adult rats under stressful conditions.6 Social defeat stress increased dimethylation of histone (H3) at the promoter of exons IV and VI of the brain-derived neurotrphic factor (BDNF) gene in the mouse hippocampus.7
These previous findings suggest that gaining further insight into the epigenetic mechanisms of neuronal plasticity in response to environmental stimuli could promote our understanding of the pathophysiology of stress-related mental disorders. In this context, we recently studied the influence of stress exposure on histone acetylation status at the promoter of exons of the BDNF gene in the rat hippocampus. In this paper, we review our latest findings regarding how different types of stress alter BDNF transcription mediated by differential usage of multiple promoters of BDNF regulated by histone acetylation status to generate region-specific expression and responses to stimuli.
INVOLVEMENT OF HISTONE ACETYLATION IN STRESS-INDUCED REDUCTION OF BDNF IN THE RAT HIPPOCAMPUS
Stress exposure is well known to lead to the onset of stress-related mental disorders such as major depression and posttraumatic stress disorder (PTSD).8-10 Although changes in gene expression under stressful condition have been reported, the precise mechanisms by which stress affects gene transcription are not fully understood.8 With regard to the pathogenesis of major depression, a series of studies showed that BDNF was closely involved in the pathophysiological and therapeutic mechanisms of this stress-related disorder.11 In fact, several studies reported that acute restraint or immobilization stress decreased the expression of BDNF in the rodent brain.12-14 Recent studies have demonstrated epigenetic regulation of BDNF gene transcription in response to external stimuli such as social defeat stress and electroconvulsive seizures.7,15 Further studies to elucidate the epigenetic regulatory mechanism of stress-induced changes in BDNF gene transcription may provide new insight into the pathophysiology of stress-related mental disorders. In this context, we examined: the influence of single immobilization stress (SIS) on the levels of total BDNF messenger RNA (mRNA) and each exon mRNA in the rat hippocampus by real-time quantitative polymerase chain reaction (PCR), BDNF protein by enzyme-linked immunosorbent assay (ELISA), and histone acetylation at each promoter of the BDNF gene by chromatin immunoprecipitation (ChIP) assay followed by real-time PCR. The detailed experimental paradigm is shown in Figure 1.
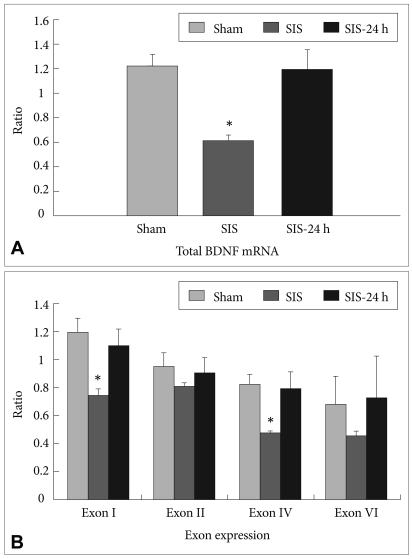
Expression of (A) total BDNF mRNA and (B) the four BDNF untranslated exons in the hippocampus of rats subjected to sham treatment (Sham), single immobilization stress (SIS), and 24 h after SIS (SIS-24h). Results are expressed as the ratio of the concentration of the target transcript to that of GAPDH (n=6, *p<0.05). BDNF: brain-derived neurotrophic factor, GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
The levels of total BDNF mRNA, exons I and IV in the hippocampus of rats subjected to SIS for 2 h were significantly lower than those of rats subjected to sham treatment (Figure 2). The levels of acetylated histone H3 at promoters I, IV, and VI were significantly reduced immediately following 2-h SIS, but the reductions were no statistically significant 24 h after the start of the 2-h SIS session (Figure 3A). There were no significant differences in the hippocampal levels of acetylated histone H4 at all 4 promoter regions among these 3 groups (Figure 3B). In addition, we examined the influence of transcriptional changes on BDNF protein levels. Significant reduction in BDNF protein levels was found only 2 h after the start of the SIS session (Figure 4).

Analysis of the levels of acetylated histone H3 (A), acetylated histone H4 (B) at each promoter region of the BDNF gene in the hippocampus of rats subjected to sham treatment (Sham), single immobilization stress (SIS), and 24 h after SIS (SIS-24 h). Results are expressed as the ratio of the concentration of the target transcript to that of "input" DNA (n=6, *p<0.05). BDNF: brain-derived neurotrophic factor.
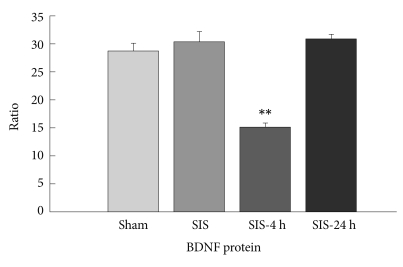
The levels of BDNF protein were measured in the rat hippocampus immediately after a single immobilization stress (SIS), 4 h after the initiation of SIS (SIS-4 h), and 24 h after the initiation of SIS (SIS-24 h). Sham: rats subjected to sham treatment only. Results are expressed as the ratio of the concentration of BDNF to that of total protein (n=6, **p<0.01). BDNF: brain-derived neurotrophic factor.

Comparison of the levels of BDNF exon mRNAs in the hippocampus after contextual fear conditioning. Data are expressed as the ratio of the concentration of the target molecule to that of GAPDH (target molecule/GAPDH) and represent the mean±SEM (10 rats per sham group and 12 rats per SPS group for total BDNF mRNA, 10 rats per group for exon I, II, IV and VI BDNF mRNA). Asterisk denotes significance at the 0.05 level. FC: fear conditioning, SPS: single prolonged stress, BDNF: brain-derived neurotrophic factor, SEM: standard error of the mean, GAPDH:glyceraldehyde-3-phosphate dehydrogenase.
The results of the present study demonstrate that SIS significantly reduces the levels of total BDNF mRNA as well as BDNF exon I-, and exon IV-containing mRNAs in the rat hippocampus, and this was accompanied by a significant decrease in the levels of acetylated H3 at the promoter of exon I, IV, and VI of the BDNF gene. Differential usage of multiple promoters of BDNF is considered to generate region-specific expression and responses to both physiological stimuli such as diurnal rhythm and exercise, and pathophysiological insults such as seizures, ischemia, and hypoglycemic coma.16-18 Recently, Nair et al.,19 using in situ hybridization, demonstrated that a SIS significantly decreased the levels of all exon-specific BDNF mRNAs in the dentate gyrus of the adult rat hippocampus. In addition, a significant reduction in the level of exon IV-specific BDNF mRNA was found in the CA3 and CA4 regions. Despite the methodological difference, the SIS-specific regulation of distinct BDNF transcripts demonstrated by Nair et al. is consistent with that in our study.
Furthermore, the finding that SIS significantly decreased acetylated H3 at the promoters of exon I and IV of the BDNF gene indicates that epigenetic mechanisms play a role in the stress-induced changes in distinct BDNF transcripts in the hippocampus. Based on our findings, it is postulated that SIS affects chromatin remodeling via enhanced levels of acetylated H3 at the promoter of the BDNF gene, and subsequently changes the levels of exon-specific BDNF transcripts in the rat hippocampus. Thus, this is the first study to show that SIS alters histone acetylation, a component of the epigenetic machinery, in the rat hippocampus.
Chronic social defeat stress has been shown to downregulate the mRNA levels of BDNF transcripts IV and VI in the mouse hippocampus, which is accompanied by significant increases in H3-K27 dimethylation at the promoter of exons IV and VI without changes in H3 acetylation status at the promoter of either exons IV or VI.7 This result taken together with our findings suggests that chromatin remodeling through histone modification could play an important role in stress-induced gene expression in vivo. We found that the levels of acetylated histone H3, but not H4, on each promoter of the BDNF gene correlated with the mRNA expression of each exon, except exon VI, in response to SIS. To our knowledge, only 2 studies have examined the regulatory effect of histone acetylation on the expression of BDNF mRNA in the rat hippocampus after an acute stimulation.15,20 In response to pilocarpine-induced status epilepticus, increased levels of exon I and IV of the BDNF gene were, at least in part, derived from the hyperacetylation of histone H4 at the P2 promoter and deacetylation of histone H4 associated with the P4 promoter.20 Likewise, the marked increase in histone H4 acetylation, but not histone H3 acetylation, at the P2 promoter of the BDNF gene correlated with an increase in total BDNF mRNA in the rat hippocampus 2 h after electroconvulsive seizure.15 In contrast with our study, these previous studies indicated the importance of histone H4 acetylation at the P2 promoter in the regulation of BDNF mRNA expression. A likely explanation for this discrepancy is that the transcriptional pathway of the BDNF gene affected by SIS is different from that in response to acute seizure.
Another interesting result derived from this study, is that the marked decreases in H3 acetylation at the promoter of exons I, IV, and VI of the BDNF gene in response to SIS spontaneously recovered 24 h after stress. Although we did not measure the activity of either HAT or HDAC in this study, recent studies indicate that the status of histone acetylation is rapidly regulated (within minutes) depending on the balance of activity between HAT and HDAC. In contrast, the status of DNA methylation was established by longer and more stable regulation (over hours to years).21,22 Thus, we postulated that plastic changes in histone modification may lead to alterations in gene expression induced by environmental stimuli. Our results indicated that the spontaneous recovery from the decreased levels of BDNF mRNA and protein 24 h after stress is, at least in part, due to plastic changes in the acetylation status of H3 at the promoters of the BDNF gene.
In summary, the present study demonstrates that stress affects transcription of the BDNF gene via histone acetylation and demethylation. Further studies examining whether stress affects other epigenetic pathways involved in regulation of the BDNF gene, such as DNA methylation, may provide additional valuable information regarding the pathophysiology of stress-related mental disorders.
INVOLVEMENT OF HISTONE ACETYLATION IN FEAR MEMORY CONSOLIDATION THROUGH THE BDNF PATHWAY
Numerous clinical studies have demonstrated that patients with PTSD exhibit long-lasting reexperience of traumatic events and avoidance of the trauma-related stimuli, even though they recognize that the traumatic events are no longer occurring. Therefore, it has recently been suggested that the impairment of fear extinction plays an important role in the development of clinical symptoms, such as reexperiencing of trauma, in PTSD.23-26
Among several genes involved in fear memory formation, BDNF has been implicated in synaptic plasticity and learning and memory. BDNF has been shown to play a critical role in the induction and maintenance of LTP as well as hippocampal-dependent learning. With regard to emotional learning, such as fear conditioning (FC), Hall et al.27 reported that BDNF mRNA expression is upregulated in the hippocampal CA1 region 24 h after contextual FC, suggesting that BDNF signaling may contribute to synaptic plasticity in this form of learning. Liu et al.28 investigated the functional role of BDNF signaling in FC using BDNF heterozygous null (BDNF+/- mice. BDNF+/- mice showed severe impairment of contextual FC, whereas tone learning remained intact. Moreover, these learning deficits could be partially reversed by chronic intraparenchymal administration of recombinant BDNF protein into the hippocampus.
Despite emerging evidence regarding the behavioral and neural mechanisms of fear acquisition and expression, to our knowledge, previous studies were undertaken only in normal rodents, but rodent models of PTSD have not yet been examined in this regard. The single prolonged stress (SPS) paradigm proposed by Liberzon et al.29,30 is considered to be an appropriate animal model of PTSD. SPS consists of 3 stages: restraint for 2 h, forced swim for 20 min, and ether anesthesia.
We examined the enhancement of fear memory acquisition using the contextual fear test in SPS rats. After a 1-week acclimatization period, rats were randomly assigned to 2 groups (SPS or sham). FC was conducted 7 days after SPS. The rats were placed in a conditioning chamber (325W×280H×500D mm) and then exposed to a 180-s conditioning context without any stimulation (i.e., a tone). Immediately after that, they received a 4-s, 0.8 mA footshock. Twenty-four hours after FC, freezing behavior was measured for 5 min. We then examined the influence of contextual FC on the hippocampal mRNA levels of total BDNF and 4 BDNF exons (I, II, IV, and VI) by RT-PCR, and we subsequently assessed BDNF protein levels by ELISA. To elucidate the mechanism by which transcription of the BDNF gene is affected in response to FC in SPS rats, we evaluated hippocampal total histone acetylation by western blotting, and histone acetylation levels at each promoter of the BDNF gene in the hippocampus was measured by ChIP assay followed by RT-PCR. Hippocampal tissues were excised following decapitation before and 2 h after FC.
There were 5 main findings: 1) SPS rats exhibited enhancement of contextual fear relative to sham rats; 2) After FC, whereas BDNF exon IV and IX mRNA levels were increased in sham rats, BDNF exon I, IV, and IX mRNA levels were increased in SPS rats (Figure 5); 3) The levels of BDNF exon I, IV, and IX mRNA, and BDNF protein were significantly higher in SPS rats than in sham rats after FC, in agreement with the enhancement of contextual fear in SPS rats (Figure 4); 4) The levels of acetylated histone H3 and H4 at the promoters of exon I and IV of the BDNF gene were enhanced more in SPS rats than in sham rats after FC, and the levels correlated with the induction of specific BDNF exon (Figure 5); 5) No significant differences were found in the global acetylated histone H3 and H4 levels in SPS rats before versus after FC; the enhanced histone H3 and H4 acetylation in SPS rats was not due to an increase in global histone acetylation.
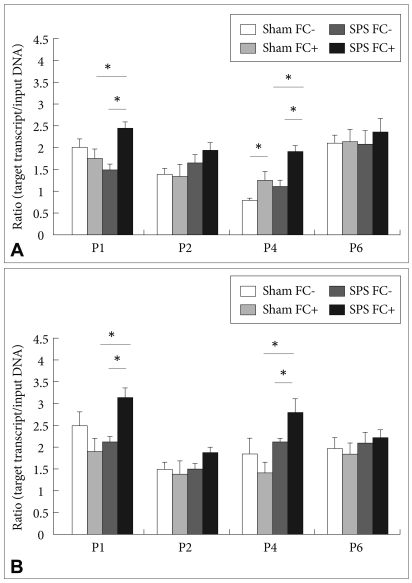
Comparison of histone H3 (A) and H4 (B) acetylation levels at BDNF promoters after contextual fear conditioning. Data are expressed as the ratio of the concentration of the target transcript to that of 'input' DNA (target transcript/'input' DNA) and represent the mean±SEM (sham before FC; 9 rats, sham 2 h after FC; 10 rats, SPS before FC; 9 rats, SPS 2 h after FC; 15 rats). SPS rats demonstrated a significant increase in the levels of acetylated histone H3 and H4 at BDNF promoters of exon I and IV after fear conditioning relative to sham rats. Asterisk denotes significance at the 0.05 level. FC: fear conditioning, SPS: single prolonged stress, BDNF: brain-derived neurotrophic factor, SEM: standard error of the mean.
To date, no other studies have examined whether contextual FC up-regulates hippocampal BDNF levels in an animal model of PTSD. In the present study, we found that SPS rats showed an increase in the levels of BDNF exon I, IV, and IX mRNA in the hippocampus 2 h after FC. The levels of BDNF exon I, IV, and IX mRNA were increased more than in sham rats 2 h after FC, in agreement with the enhanced fear responses. The BDNF gene consists of nine 5' noncoding exons each linked to individual promoter regions and a 3' coding exon (IX), which codes for the BDNF preprotein amino acid sequence.30,31 Several recent studies have examined differential usage of BDNF non-coding exons during consolidation of fear learning.3,32,33 In these earlier studies, BDNF exons I and IV were transcriptionally upregulated in the amygdala during consolidation of fear learning.32,33 Bredy and colleagues34 showed that extinction of conditioned fear is accompanied by a significant increase in histone H4 acetylation at the promoter of exon IV of the BDNF gene and an increase in exon I and IV mRNA of the BDNF gene in the prefrontal cortex of mice. Lubin and colleagues3 reported that only BDNF exon IV was transcriptionally upregulated in the CA1 region of the hippocampus during consolidation of fear learning. Our results are largely consistent with these previous findings, although there are some differences arising presumably from differences in the brain regions studied or in the experimental designs. Taken together with previous studies, our results support that exon IV-containing BDNF transcripts plays a pivotal role in the enhanced expression of total BDNF mRNA during the consolidation of contextual fear.
Several studies have recently reported that chromatin-modifying mechanisms are involved in learning and memory processes in adult neurons.3,5,35 For example, Levenson et al.35 showed that whereas histone H3 was acetylated following contextual FC in the hippocampus, histone H4 was acetylated following a latent inhibition protocol for contextual FC. Lubin et al.3 investigated histone modifications at specific BDNF promoters after contextual FC. They observed a selective increase in histone H3 acetylation at the BDNF gene promoter of exon IV, and a selective decrease in histone H4 acetylation at the BDNF promoter of exon II. In our study, SPS rats demonstrated a significant increase relative to sham rats in the levels of acetylated H3 and H4 at BDNF promoters of exons I and IV after FC. In addition, this selective increase in histone acetylation at the promoters of exons I and IV were consistent with up-regulation in exon I and IV mRNA transcription associated with FC. Interestingly, there were no alterations in the total levels of H3 and H4 histone acetylation after FC. These findings indicate that the selective increase in histone acetylation at promoters of exons I and IV is not due to global histone acetylation, raising the possibility that histone acetylation occurs with relative gene specificity. There have been no previous reports on the participation of histone H4 acetylation in the consolidation of contextual fear. Although it remains unknown whether histone H3 and H4 acetylation play different roles in fear consolidation, the fact that SPS increased acetylation of H3 as well as H4 at the BDNF promoters of exons I and IV after FC, indicates that coordinate enhancement of histone acetylation may play a role in the marked enhancement of contextual fear memory in SPS rats.
In summary, this is the first study to demonstrate in an animal model of PTSD that contextual fear is associated with transcription of the BDNF gene in the hippocampus via changes inhistone acetylation. It is postulated that the higher induction of BDNF in response to contextual FC in SPS rats may contribute to the difference in freezing between SPS and sham-treated rats. Our study suggests that enhanced levels of BDNF through histone acetylation during fear memory consolidation is, at least in part, associated with long-lasting fear memory in patients with PTSD.