The Effects of Venlafaxine and Dexamethasone on the Expression of HSP70 in Rat C6 Glioma Cells
Article information
Abstract
Objective
The present study aimed to determine the intracellular action of the antidepressant, venlafaxine, in C6 glioma cells using heat shock protein 70 (HSP70) immunocytochemistry and HSP70 Western blots; HSP70 is known to be associated with stress and depression.
Methods
The extent of HSP70 expression was measured after rat C6 glioma cells were treated with 1) dexamethasone only, 2) venlafaxine only, 3) simultaneous venlafaxine and dexamethasone, or 4) dexamethasone after venlafaxine pretreatment. Dexamethasone (10 µM, 6 hours) did not affect the level of HSP70 expression relative to control.
Results
Short-term (1 hour) venlafaxine treatment significantly increased the level of HSP 70 expression. Simultaneous long-term (72 hours) venlafaxine and dexamethasone treatment significantly reduced the level of HSP70 expression. Dexamethasone treatment administered following long-term (24 and 72 hours) pretreatment with venlafaxine also significantly reduced the level of HSP70 expression.
Conclusion
Short-term treatment with venlafaxine increases the expression of HSP70, but prolonged treatment with dexamethasone suppresses the venlafaxine-induced expression of HSP70. These findings suggest that HSP70 and dexamethasone play a significant role in the pathophysiology of depression.
Introduction
During emergencies, there is a dramatic increase in the production of heat shock protein (HSP) in all organisms, the function of which is to repair damage.1 HSP functions as a molecular chaperone bonding to the exposed hydrophobic portions of proteins to prevent their aggregation and to maintain their original conformation. Under nonstressful conditions, HSP forms a complex with the transcription factor monomer heat shock transcription factor (HSF), which exists in the cytoplasm and the nucleus, by one-to-one bonding. However, in stressful conditions the degree of misfolding among proteins increases in cells, causing HSP to be separated from HSF and to bond with the misfolding protein. HSF separates from the HSP conglomerate as a trimer, migrates into the nucleus, bonds to the heat shock element located in the heat shock gene promoter, and then activates heat shock gene transcription. As a result, when the expression of free HSP increases, HSF is separated from the deoxyribonucleic acid and contributes as a monomer to the increased formation of the HSP-HSF complex. These processes control the expression of HSP.2
The 70-kDa HSP (HSP70) is known to be associated with the psychopathology of depression. Depletion of HSP70 messenger ribonucleic acid has been observed in some depressed patients, but not in control subjects or in patients with other diseases.3 HSP70 is also known to control the glucocorticoid receptor (GR), which is known to play a role in depression.4,5
HSP70, which is observed in abundance in healthy cells, also functions as a molecular chaperone under non-stressful conditions. When the cell is exposed to stress, a massive amount of HSP70 is synthesized in an attempt to maintain the cell's homeostasis.6,7 HSP70 is the first HSP to be synthesized, and is important for the activation of structurally unstable GRs.4,5 It has been reported that in major depressive disorders,3,8 the allele-specific abnormal transcription of the HSP70 gene on chromosome 6 underlies the altered stress and/or immune response, and the functions of antidepressants9,10 by preventing the neurotoxicity of N-methyl-D-aspartate receptor antagonists.
Depressive patients exhibit increased cortisol levels in the plasma, urine, and cerebrospinal fluid, a hyperreaction of cortisol to adrenocorticotrophic hormone, and hypertrophy of the pituitary and adrenal glands.11-13 The most consistent finding in depressed patients is hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis, as demonstrated using the dexamethasone/corticotropin-releasing factor test, which is considered to be the most sensitive measure of HPA axis hyperactivity.14 When the concentration of glucocorticoids is kept high for a prolonged period of time, symptoms of neurotoxicity develop that are thought to result from the effacement of pyramidal neurons in the hippocampus and atrophy of the dendrites.15-17
Currently, monoamine reuptake inhibitors have been used as antidepressants for the treatment of depression. In particular, venlafaxine is used as the inhibitor of both serotonin and norepinephrine. The primary function of venlafaxine is to protect the transport of serotonin and norepinephrine at the synapse, thus increasing the concentration of both monoamines within the synapse. However, even though the level of both monoamines in the synapse increase immediately after the administration of venlafaxine, there is a 2- to 6-week delay in its antidepressant effects.18 It has been suggested that this delay results from changes in postreceptor signaling19-21 or interactions with HSP70.9,10
In the study presented here, we investigated mechanisms other than the monoamine system, such as receptors and transporters of serotonin and norepinephrine, as the possible mechanisms of the antidepressant effect of venlafaxine in depression. More specifically, we evaluated the relationship between dexamethasone and venlafaxine, which affect the level of HSP 70 expression in rat C6 glioma cells. These cells, which have high levels of GRs but lack serotonin and norepinephrine transporters, react to the antidepressant similarly in vivo and in vitro by, for example, exhibiting an increase in adenylyl cyclase activity.22-24 The expression of HSP70 was investigated using immunoblotting after groups of rat C6 glioma cells were each treated with 1) with dexamethasone only, 2) venlafaxine only, 3) with simultaneous venlafaxine and dexamethasone, or 4) dexamethasone after venlafaxine pretreatment. The objective of the third procedure was to determine the effect of venlafaxine on HSP70 under conditions of clinical depression, and the objective of last procedure was to determine the prophylactic effect of venlafaxine treatment on HSP70 under the conditions of depression.
Methods
Materials
Rat C6 glioma cells were obtained from ATCC (Manassas, VA, USA), dexamethasone was obtained from Sigma-Aldrich (St. Louis, MO, USA), and the antibody for HSP70 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Venlafaxine was provided by Wyeth Korea (Seoul, Korea). All other chemicals were purchased from Sigma-Aldrich.
Cell cultures and reagent treatments
C6 glioma cells were cultivated in Dulbecco's modified essential medium (DMEM)(Gibco BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum (Gibco BRL) in a 5% CO2 cultivator at 37℃. The DMEM culture medium was changed every 48 hours and cells were cultivated to the stable growth stage. Dexamethasone was dissolved in 95% ethanol to a concentration of 2.55 mM and then stored at -20℃. The ethanol in 0.25 mL of stored solution was evaporated in Nunc-Immuno plates (Vangard International, Neptune, NJ, USA) just before the experiment. Venlafaxine was dissolved into a mixture of 10% ethanol and sterilized water, filtered, and then diluted prior to use. The concentrations of dexamethasone (10 µM) and venlafaxine (10 µM) were set at optimal points at which it is know that apoptosis does not occur, as determined in preliminary cultivating experiments25-27 using various concentrations of the drugs (5, 10, 50, and 100 µM). Each of the following procedures was repeated six times.
To enable determination of the expression of HSP70 in the rat C6 glioma cells after treatment with dexamethasone, the culture solution was replaced with a new solution containing dexamethasone (10 µM) when the cells in the incubator showed 85% growth. The cells were treated with dexamethasone for 6 hours and the expression of HSP70 was measured using an anti-SP70 monoclonal antibody (anti-HSP70mAb).
To investigate the effect of venlafaxine treatment on HSP70 expression, the culture solution was replaced with one that contained only venlafaxine (10 µM) when the cells showed 85% growth. Each group was treated for 1, 6, 24, and 72 hours. The expression of HSP70 at each of these time points was investigated using anti-HSP70mAb. The effects of simultaneous treatment with venlafaxine and dexamethasone were determined by replacing the culture solution with one containing venlafaxine (10 µM) and dexamethasone (10 µM) when the cell showed 85% growth. Each group was treated for 1, 6, 24, and 72 hours. The expression of HSP70 at each of these time points was determined using anti-HSP70mAb.
The effects of pretreatment with venlafaxine on the action of dexamethasone were determined by first replacing the culture medium with one containing venlafaxine (10 µM) and incubation them for 1, 6, 24, and 72 hours. The culture medium was then replaced with one containing dexamethasone (10 µM) and treated for a further 6 hours. The expression of HSP70 was then evaluated as before.
Protein extraction
Cells were washed in a 100-mm-diameter dish with phosphate-buffered saline and then gathered by centrifugation at 2,000-3,000 rpm for 5 min. A 400-µL volume of Pro-Prep (iNtRon Biotechnology, Seongnam, Korea) was added to samples of 5×106 cells and the suspension stirred thoroughly. Cells were dissolved in ice for 20 min and the suspension then centrifuged again at 13,000 rpm at 4℃ for 5 min. The resulting supernatant was poured into a 1.5-mL tube and stored at -20℃ until protein quantification and immunoblotting.
Protein quantification
The concentration of proteins in each sample was measured using Bradford's method (Bio-Rad Protein Assay Kit, Bio-Rad Laboratories, Hercules, CA, USA). A working solution was made from diluted Bradford reagent in distilled water at a ratio of 1 : 5. We prepared 10 µL aliquots of 1, 0.5, 0.1, 0.05, 0.01, and 0 mg/mL of bovine serum albumin standard, poured each standard into 96-well plates, and then added 200 µL of working solution. After leaving the solution at room temperature for 5 min, absorbance was measured on an enzyme-linked immunosorbent assay reader at a wavelength of 595 nm. Protein concentration was then estimated using a standard curve.
Western blot analysis
Immunoblotting was performed to determine HSP70 expression by using anti-HSP70mAb against cell extracts. The same amount of protein was added to Laemmli sample buffer [62.5 mM Tris-HCl, pH 6.8; 2% sodium dodecyl sulfate (SDS); 10% glycerol; 0.5% β-mercaptoethanol; and 10 µg/mL bromophenol blue], and the proteins were denatured by boiling for 5 min. Each protein was separated on a 10% polyacrylamide gel using SDS-polyacryamide gel electrophoresis, and then electrotransferred onto nitrocellulose membranes. After blocking with 5% nonfat dry milk in Tris-buffered saline Tween 20 (TBST) solution (20 mM Tris-HCl, pH 7.6; 500 mM NaCl; and 0.1% Tween 20), the membranes were incubated with 200 µg/mL of anti-HSP70mAb (diluted to 1 : 2000) at room temperature for 60 min, followed by horseradish-peroxidase-conjugated antimouse IgG (diluted to 1 : 1000) for 60 min. After washing twice in TBST (15 min each), the reactions were detected using an enhanced chemiluminescence detection system (Amersham Life Science, Buckinghamshire, UK). The concentration of HSP70 was quantified with QuantityOne software (Bio-Rad Laboratories).
Statistical analysis
Data were expressed as mean±standard error of measurement (SEM) values, and the level of statistical significance was set at p<0.05. The analyses were performed using the Statistical Package for the Social Sciences (SPSS) 12.0 for Windows (SPSS, Chicago, IL, USA). Analysis of variance was used to distinguish the different patterns of HSP70 expression according to chemical treatments. The degree of statistical significance between groups was investigated using paired-samples t-tests.
Results
Effect of dexamethasone on the expression of HSP70
The expression of HSP70 was slightly lower in the dexamethasone-treated group than in the control group, but the difference was not statistically significant (Figure 1).
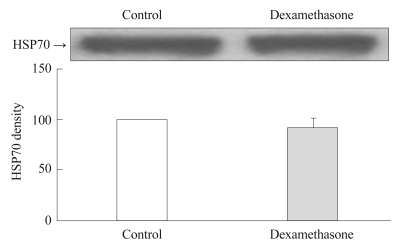
Effect of dexamethasone on HSP70 expression in rat C6 glioma cells. Cells were treated with dexamethasone for 6 hours. There was no difference in the extent of HSP70 expression between control and the dexamethasone-treated group. Values of HSP70 density are represented as the mean±SEM (n=6) of the percent change from 100% control group. HSP70: heat shock protein 70, SEM: standard error of measurement.
Effect of venlafaxine on the expression of HSP70
The expressions of HSP70 in the experimental group were significantly higher 1 hour after treatment with venlafaxine compared to the control group (p=0.027). The expression of HSP70 tended to decrease after 24 hours (Figure 2).
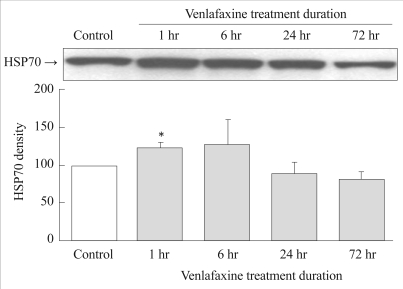
Effect of venlafaxine on HSP70 expression in rat C6 glioma cells. Cells were treated with venlafaxine for 1, 6, 24, and 72 hours, whereas the control group was not treated with venlafaxine. Complared to the control group, 1 hour treatment of venlafaxine showed significantly increased expressions of HSP70. Values of HSP70 density are represented as the mean±SEM (n=6) of the percent change from 100% control group. *p<0.05 in comparison to the control group. HSP70: heat shock protein 70, SEM: standard error of measurement.
Effects of simultaneous treatment with venlafaxine and dexamethasone on the expression of HSP70
Simultaneous treatment with venlafaxine and dexamethasone for 1 hour resulted in an increase in the expression of HSP 70 compared to controls. However, the expression of HSP70 decreased dramatically thereafter with time, so that after 72 hours of treatment the expression of HSP70 had significantly decreased compared to the control group (p=0.001)(Figure 3).
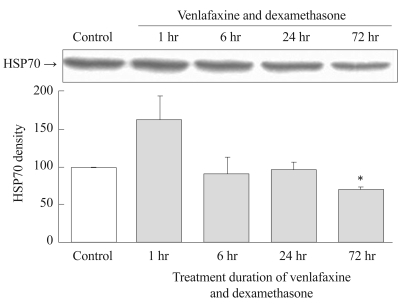
Effect of simultaneous treatment with venlafaxine and dexamethasone on HSP70 expression in rat C6 glioma cells. Cells were treated with venlafaxine and dexamethasone for 1, 6, 24, and 72 hours, whereas the control group was treated neither with venlafaxine nor with dexamethasone. Compared to the control group, 72 hours treatment of venlafaxine and dexamethasone group showed significantly decreased expressions of HSP70. Values of HSP70 density are represented as the mean±SEM (n=6) of the percent change from 100% control group. *p<0.05 in comparison to the control group. HSP70: heat shock protein 70, SEM: standard error of measurement.
Effect of dexamethasone on the expression of HSP70 in venlafaxine-pretreated cells
The effect of dexamethasone on the expression of HSP70 was dependent on the duration of venlafaxine pretreatment. For the groups pretreated with venlafaxine for 1 and 6 hours, no significant differences in HSP70 expression were observed compared to the control. On the other hand, groups that were pretreated with venlafaxine for 24 hours (p=0.003) and 72 hours (p=0.002) exhibited a significant reduction in HSP70 expression by dexamethasone compared to the control group (Figure 4).
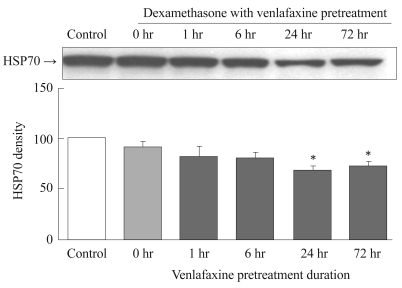
Effect of dexamethasone on HSP70 expression in venlafaxine-pretreated rat C6 glioma cells. Cells were pretreated with venlafaxine for 1, 6, 24, and 72 hours, and then treated with dexamethasone for 6 hours. 0 hr group was treated only with dexamethasone for 6 hours. Compared to the control group, the groups which were pretreated with venlafaxine for 24 and 72 hours showed significantly decreased expressions of HSP70. Values of HSP70 density are represented as the mean±SEM (n=6) of the percent change from 100% control group. *p<0.05 in comparison to the control group. HSP70: heat shock protein 70, SEM: standard error of measurement.
Discussion
The two main findings of this study are as follows: 1) HSP 70 expression increases soon after venlafaxine treatment, with or without dexamethasone treatment; and 2) simultaneous treatment with dexamethasone and venlafaxine or treatment with dexamethasone following pretreatment with venlafaxine results in a subsequent reduction in HSP70 expression.
The early increase in HSP70 expression found in this study (after 1 hour of treatment) is pertinent to several recent studies that showed tricyclic antidepressant-induced apoptosis in many cells.28-30
One of the selective serotonin reuptake inhibitors, fluoxetine, also induces apoptosis in C6 glioma cells.28 The results of our study raise the possibility that venlafaxine is a stressor in the early stage of treatment (1 hour), even though it has been shown that it does not induce apoptosis at the low concentrations used in this study.
The design of our study did not allow us to determine the factor responsible for the decline of HSP70. Further research is required to determine the exact cause of the lowered HSP70 expression. However, there are a few possible mechanisms that could underlie a reduction in HSP70 expression induced by the chronic administration of the antidepressant venlafaxine. Firstly, venlafaxine may reduce the activity of HSF by inducing mitogen-activated protein kinase (MAPK). Some studies31,32 have shown that MAPK is a negative regulator of HSF, and another33 has shown that extracellular signal-regulated kinase, which is one of the MAPKs, inactivates HSF. Khawaja et al.27 reported increases in the activity of MAPK pathways such as p90 ribosomal S6 kinase (p90Rsk), phosphorylated cyclic AMP response element-binding protein, and phosphorylated ELK-1 after 3 days of treatment with venlafaxine in C6 glioma cells. Therefore, the venlafaxine-induced increased activity of MAPK pathways may inhibit HSF activity and result in a reduction of HSP70 expression. Secondly, venlafaxine induces the translocating mechanism of GRs and the resultant decrease in HSP70 expression. Various types of antidepressant, such as fluoxetine, milnacipran, and clogyline, are known to cause GR translocation.34 Translocation of GRs into the nucleus inhibits the activity of HSF. The level of HSP70 expression could be decreased by the negative feedback of inhibiting trimerization of HSF in the process of GR translocation.
Three recent studies involving various rat tissues27,35,36 support the antidepressant effect of venlafaxine, and also suggest several mechanisms of action. The findings of one of those studies suggest that some of the centrally mediated benefits of venlafaxine in depression are due to its intracellular properties, especially on the neuroglial circuitry and MAPK/p90Rsk-dependent pathways at an early stage.27 One of the other studies has suggested that quetiapine and venlafaxine both target the hippocampus of stressed rats by enhancing hippocampal resilience, which may be impaired in patients with schizophrenia or depression.35
In summary, the intracellular action of the antidepressant venlafaxine was studied in rat C6 glioma cells using HSP70 immunocytochemistry and HSP70 Western blots; HSP70 is known to be associated with stress and depression. We observed that the application of 10 µM dexamethasone for 6 hours did not affect the level of HSP70 expression relative to control. This finding was contrary to our expectations and is inconsistent with a previous report concluding that dexamethasone is an inducer of the heat shock response.37 Short-term (1 hour) treatment with venlafaxine significantly increased the level of HSP 70 expression, whereas long-term (72 hours) simultaneous treatment with venlafaxine and dexamethasone significantly reduced.
Venlafaxine increases the expression of HSP70 in the short term, which might be due to it acting as a stressor. However, prolonged treatment with dexamethasone suppresses the expression of HSP70, possibly due to the induction of MAPK and GR translocation.
An in vivo experiment is needed to validate the results of this study. Nevertheless, this work represents an important in vitro model that could be used to screen potential novel antidepressants. Such a model would considerably benefit the field and also attempts by the drug industry to identify such compounds. The present study suggests that the treatment of depression with venlafaxine plays a role in preventing stress, and provides fundamental data to explain the pathophysiological mechanisms underlying depression.
Acknowledgments
This paper was supported financially by Konkuk University (Seoul, Korea) in 2006.