No Evidence for an Association between Dopamine D2 Receptor Polymorphisms and Tardive Dyskinesia in Korean Schizophrenia Patients
Article information
Abstract
Objective
Tardive dyskinesia (TD) is a long-term adverse effect of antipsychotic. Dopaminergic activity in the nigrostriatal system have been proposed to be involved in development of TD and dopamine D2 receptors (DRD2) has been regarded as a candidate gene for TD because the antipsychotics have potent antagonism DRD2. This study was aimed to find the relationship between DRD2 gene and antipsychotic-induced TD.
Methods
We evaluated whether 5 DRD2 single nucleotide polymorphisms (-141Cins>del/TaqID/NcoI/Ser311Cys/TaqIA) are associated with antipsychotic-induced TD in 263 Korean schizophrenia patients with (n=100) and without TD (n=163) who were matched for antipsychotic drug exposure and other relevant variables. Haplotype analyses were also performed.
Results
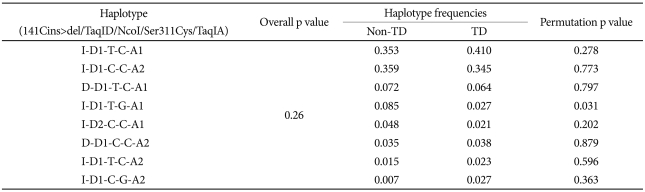
None of 5 polymorphisms were found to be significantly associated with TD and with TD severity as measured by Abnormal Involuntary Movement Scale. Overall haplotype (-141Cins>del/TaqID/NcoI/Ser311Cys/TaqIA) frequency was also not significantly different between TD and non-TD groups, although one rare haplotype (I-D1-T-G-A1) showed significantly different frequency between TD and non-TD groups (2.7% vs. 8.5%, respectively, p=0.031).
Conclusion
The present study does not support that DRD2 gene may be involved in TD in the Korean population, although further studies are warranted.
INTRODUCTION
Tardive dyskinesia (TD) is one of the most serious adverse effects of antipsychotic medication. The typical symptoms of TD are involuntary movements of the orofacial musculature. However, the trunk and extremities may also be affected. TD is known to appear in 20-30% of schizophrenic patients treated with typical neuroleptics.1,2 Although the risk of TD is reportedly lower for newer atypical antipsychotics than for typical antipsychotics, it has not been ascertained whether there has been an associated decrease in the prevalence of TD.3
Only a certain portion of patients exposed to long-term treatment with antipsychotic drugs develop TD, which suggests that individual susceptibility, such as genetic factors, is important. Genetic vulnerability to the development of TD has been suggested based on the results of studies in animals4 and humans (i.e., families).5 Several biological mechanisms underlying TD have been hypothesized, including dopamine receptor supersensitivity,6 the dysfunction of the serotonergic system,7 gamma-aminobutyric acid insufficiency,8 and disturbances in antioxidative protection.9 However, the pathophysiology of TD is not well understood.
Dopamine receptor hypersensitivity hypothesis is based on several observations.10 Neuroleptic-induced dyskinesia in a non-human primate showed significantly decreased dopamine turnover in the caudate and substantia nigra.11 The dopamine D2 recepto (DRD2) density in the basal ganglia after continuous antipsychotic treatment of rats also increased.12-14 In addition, Acute behavioral responses to the administration of dopamine-agonist following the withdrawal of antipsychotics have been reported in rat models.15-17 Many studies have revealed that typical antipsychotics that generally have higher affinities for DRD2 is more likely to develop TD compared to atypical antopsychotics which have relatively lower affinities for it.18-20 Thus, variation in DRD2 function and expression may contribute to the risk of antipsychotic-induced TD.
Some previous studies reported the relationship between DRD2 variants and TD. Chen et al.21 reported that the frequency of the TaqI A2/A2 genotype of the DRD2 was higher in Taiwanese female schizophrenic patients with TD than those without TD. Recently, there was the report that allelic and haplotypic association between the DRD2 TaqIA/TaqIB variants and TD in a Chinese population.22 Zai et al.23 analyzed twelve DRD2 polymorphisms in TD and found that haplotypes containing rs6275 (NcoI) and rs6277 were associated with TD. However, the association between DRD2 and TD was not confirmed by other studies.24-29 Recently a meta-analysis reported the positive association between DRD2 TaqIA and TD.30 However, the odds ratios (ORs) were only ranged from 1.1 to 1.5. These inconsistent findings suggest that the relationship remains controversial.
These contradictory results prompted us to investigate whether the DRD2 gene plays a role in the susceptibility to TD in Korean schizophrenic patients. In the present study, we investigated the possible association between 5 polymorphisms of the DRD2 gene and TD.
METHODS
Subject and assessment
Two hundred and sixty three unrelated Korean schizophrenic patients participated in this study (100 TD and 163 non-TD patients), all of whom were inpatients enrolled from the three collaborating hospitals of Korea University Hospital. This study was approved by the Ethics Committee of the Korea University Hospital, and written informed consents were obtained from all the participants. All patients met the criteria for a diagnosis of schizophrenia as determined by board-certified psychiatrists using the Korean version of the Structured Clinical Interview for the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders.31 Subjects with significant comorbid neurological illness, mental retardation, substance abuse, and other major psychiatric comorbidities were excluded.
The participants consisted of schizophrenic patients with (n=100) and without (n=163) TD who were matched for antipsychotic drug exposure and other relevant variables. All of the non-TD had been treated with typical antipsychotics for at least 10 years. However, our sample included TD patients who had taken atypical antipsychotics (n=23) and who had been treated for less than 10 years (n=18). We applied these different inclusion criteria since the occurrence of TD in patients treated for less than 10 years is indicative of a higher genetic susceptibility.
All of the subjects had taken the stable dosage of antipsychotics for at least 3 months before TD was assessed. TD was diagnosed based on the Abnormal Involuntary Movement Scale (AIMS),32,33 which measures the severity of involuntary movements in seven domains on a scale from 0 to 4. Subjects were considered to have TD when they had two or more 2-point ratings, or one or more 3-point ratings in the first seven items of the AIMS. Psychopathology was measured using the Positive and Negative Syndrome Scale.34 Other findings from these subjects have been reported previously.35-40
Genotyping
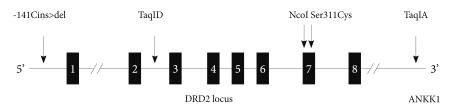
We chose 5 single nucleotide polymorphisms (SNPs) [-141 Cins>del (rs1799732), TaqIA(rs1800497), TaqID(rs1800498), Ser311Cys (rs1801028), NcoI (rs6275)] from DRD2 (Figure 1), which spans about 270 kb where -141Cins>del is in the promoter region, TaqID is intron 2, rs6275 is in exon 7. TaqIA is in the 3'-untranslated region, which is in fact located in a novel gene, untitled X-kinase gene.41 The SNP selection was based on the previous studies and minor allele frequency in Asian population. Blood samples (5-10 mL) were collected into EDTA tube, and genomic deoxyribonucleic acid (DNA) was isolated using NucleoSpin Genomic DNA Extraction Kit (Macherey-Nagel, Germany) according to standard procedures. Genotyping of TaqIA and TaqID was modified on the basis of Kaiser et al.,42 while analysis of -141Cins>del was modified according to Jönsson et al.43 The Ser311Cys polymorphism was typed as previously described.44 NcoI polymorphism (rs6275) was genotype as described by Sarkar et al.45
Statistical analysis
The presence of Hardy-Weinberg equilibrium was tested with the χ2 test for goodness of fit. Categorical data were also analyzed by using the χ2-test and differences for continuous variables were evaluated by using the Student's t-test or analysis of covariance. The cutoff p value was set at 0.05. We performed haplotype-based case-control analysis of the 5 SNPs. Haplotype and linkage disequilibrium (LD) analyses were performed using the software SNPAlyze version 7 (DYNACOM Co., Ltd. Yokohama, Japan). Overall distribution of haplotypes was analyzed using 2 X m contingency tables, with a p value of <0.05 considered to indicate statistical significance. The p value of each haplotype was determined using chi-square analysis, the permutation method, and SNPAlyze version 7. Pairwise LD patterns were evaluated using D'. The power analysis was performed with using of G*Power 3.1.2 software.46
RESULTS
The genotypic frequencies of the five DRD2 polymorphisms were within the Hardy-Weinberg equilibrium. Table 1 shows the demographic characteristics (age and sex) as well as total AIMS scores and TD diagnoses with genotypes and allele frequencies of the 5 SNPs in DRD2. This study had a power of approximately 0.70 to detect a small effect size of 0.20 and 0.98 to detect a medium effect size of 0.30 in the genotype frequencies (n=263 for total sample). There was no significant difference in age, gender, and total AIMS score among the genotype groups. There was also no significant difference in the distribution of genotypic frequencies among patients with TD and without TD (TaqIA: chi-square=0.91, df=2, p=0.634; TaqID: chi-square=2.56, df=2, p=0.279; NcoI: chi-square=0.23, df=2, p=0.892; Ser311Cys: Fisher's exact test, two tailed, p=0.326; -141Cins>del: chi-square=0.78, df=2, p=0.677) (Table 1). In addition, there is no significant difference in the distribution of allele frequencies between patients with TD and without TD (TaqIA: Fisher's exact test, two tailed, p=0.588; TaqI B: Fisher's exact test, two tailed, p=0.121; NcoI: Fisher's exact test, two tailed, p=0.857; Ser311Cys: Fisher's exact test, two tailed, p=0.352; -141 Cins>del: Fisher's exact test, two tailed, p=0.892) (Table 1).

Statistical analyses on demographics as well as total AIMS scores and TD diagnoses with genotypes and allele frequencies of the 5 SNPs in DRD2
Haplotype analysis
Seventeen haplotypes were found in the present study (data not shown), 9 of which had a frequency of more than 1% (Table 2). The most frequent two haplotypes were I-D1-T-C-A1 and I-D1-C-C-A2 (36.7% and 35.5%, respectively). Overall haplotype (-141Cins>del/TaqID/NcoI/Ser311Cys/TaqIA) frequency was also not significantly different between TD and non-TD groups, although one rare haplotype (I-D1-T-G-A1) showed significantly different frequency between TD and non-TD groups (2.7% vs. 8.5%, respectively, p=0.031).
DISCUSSION
Previous studies showed inconsistent results in the association between DRD2 polymorphisms and TD. Zai et al.23 explained these reasons as different polymorphisms used in many of the studies, few haplotype analyses, different ethnic backgrounds, small sample size, and the use of dichotomous TD occurrence for the analyses. Thus, in our study, haplotype analyses as well as SNP analyses were used, and only Korean subjects participated in our study. In addition, most analyzed DRD2 polymorphisms except NcoI were used. As a result, none of 5 polymorphisms were found to be significantly associated with TD and with TD severity as measured by AIMS. Haplotype distributions were also found to be not significantly different between TD and non-TD groups.
On the contrary, it was reported that A2/A2 genotype and A2 alleles of TaqIA were significantily associated with TD in Taiwanese females.21 Furthermore, the results from the meta-analysis suggest that TaqIA is associated with TD. However, the relatively low OR suggested that it is consistent with the idea of contributions of multiple genetic variants in complex phenotypes.30 Additionally, TaqIA association with TD could not be replicated in several study,23,29 in which the authors suggested that the previous positive finding with TaqIA could be due to higher LD in the 3' portion of DRD2. They also assumed this polymorphism is located 10 kb upstream of the 3' non-coding region of the gene. Other results except NcoI from our study are consistent with most previous studies in that TaqID, Ser-311Cys, -141Cins>del polymorphisms were found to be not significantly associated with TD.23,26-28,30,47,48 Especially, the results from the meta-analysis showed that the -141Cins>del alleles and genotypes were not associated with TD although the sample size was so small.30
There are several limitations to generalization of the results of this study. First, although anticholinergics and benzodiazepines have been reported to be beneficial in some cases of TD, in this study, we could not control those drugs. Secondly, we cannot exclude presence of population stratification bias. However, we do not think that stratification bias should be considered seriously in our sample because the Korean population is characterized by a genetic homogeneity.49 Third, the relatively small sample size limits the generalizationabilty of our findings. Because our sample size is relatively small, our data cannot exclude the possibility that DRD2 polymorphism has an influence on susceptibility to TD. Taking these limitations into account, further investigations involving additional genes and markers and larger samples are warranted to fully understand the genetic pathophysiology of TD.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-E00333).