Whole Brain Voxel-Wise Analysis of Cerebral Retention of Beta-Amyloid in Cognitively Normal Older Adults Using 18F-Florbetaben
Article information
Abstract
Objective
Recently developed 18F-labelled amyloid beta (Aβ) positron emission tomography (PET) tracers have demonstrated potentials to enable more prevalent application of amyloid imaging in the clinical setting. The aim of this study is to demonstrate cerebral retention of Aβ in cognitively normal older adults, by implementing voxel-based analysis on images acquired from 18F-Florbetaben amyloid PET.
Methods
Fifty cognitive normal elderly subjects were recruited and included in the study. Demographic data and cognitive measurements were collected. Magnetic resonance imaging (MRI) and 18F-Florbetaben PET data were obtained followed by whole brain voxel-based analysis.
Results
Compared to the florbetaben (FBB) (−) counterpart, FBB (+) showed significantly higher Aβ deposition in the brain regions comprising anterior cingulate, middle cingulate, posterior cingulate and precuneus (family wise error corrected p<0.05). There was no significant correlation between amyloid retention and cognitive functions.
Conclusion
Our results confirms previous results regarding Aβ deposition by using 18F-Florbetaben, demonstrating potentials in application of 18F-Florbetaben PET imaging in clinical settings.
INTRODUCTION
Alzheimer disease (AD) is a neurodegenerative disease notorious for its chronic and debilitating course, and it is strongly correlated with cognitive impairments and behavioral disturbances. Among the several pathophysiological causes of AD, cerebral retention of toxic amyloid-β (Aβ) peptides in soluble or intraneuronal forms has been repeatedly suggested, with amyloid plaques known to provoke synaptic injury and neurodegeneration associated with AD.1
Introduction of amyloid positron emission tomography (PET) has contributed to the unraveling of the underlying pathological mechanisms of AD.2 To date, the most widely used and studies Aβ PET tracer has been, 11C-labelled Pittsburgh compound-B (PiB).3 However, considering short half-life of carbon radioisotope (20 min), use of PiB is largely restricted, hindering it from active application in clinical settings.4 In these regards, 18F-labelled Aβ PET tracers longer half-life of 110 min (florbetaben, florbetapir, flutemetamol), have been discovered and gained US Food and Drug Administration approval for use in clinical practice.4 Among the 3 F labeled Aβ tracers, florbetaben (FBB) was the first 18F-labeled Aβ tracer studied in humans.4 Several prior works showed high sensitivity and specificity (90% sensitivity and 80% specificity) in discriminating AD from the normal controls.5 However, as compared to the studies using PiB, there is no comparison study on cerebral retention pattern between the cognitively intact elderly according to their Aβ retention status. As Aβ protein is known to accumulate for 10–15 years before the emergence of cognitive impairments in AD, measuring Aβ retention pattern in the cognitively normal older adults would help to understand more sophisticated pathophysiological process underpinning AD trajectory.6 In this study we used whole brain voxel wise analysis to measure Aβ retention pattern in cognitively normal older adults. As compared to region of interest (ROI), the benefits of the voxel wise analysis is to measure whole brain Aβ without a priori consumption. We hypothesized that the retention patterns will differ between FBB (+) and (−) group in the posterior cingulate (PCC), precunes (PRC), anterior cingulate (ACC), lateral temporal (LT) and frontal cortex (FRC) as in the previous voxel based study using the PiB.7
METHODS
Subjects
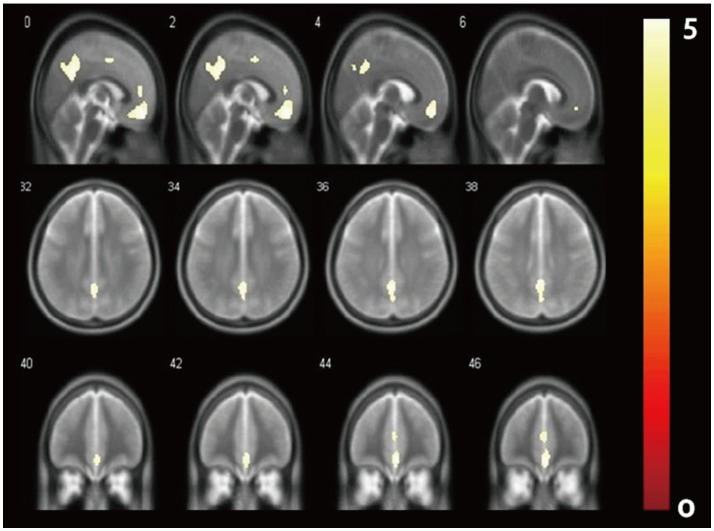
In this study, 50 elderly participants with intact cognition were recruited from the community advertisements and from the Catholic Geriatric Neuroimaging Database. The inclusion criteria of the subjects were: 1) subjects aged >60 years, 2) and Clinical Dementia Rating=0,8 and 3) Mini-Mental Status Examination score >27. Participants with any of the neurological, medical, or psychiatric diseases were excluded from the study.
The cognitive functions were tested using the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD-K).9
PET acquisition
Combined PET/CT in-line systems (Gemini TF, Philips Healthcare, Best, The Netherlands) were used to acquire all data. An average of 300 MBq FBB was injected intravenously, and the scan was initiated 90 minutes later. A low dose CT scan was performed for attenuation correction and was immediately followed by PET imaging in three-dimensional mode for 20 minutes. Fixation of the subject's head was done with a head holder to minimize motion artifacts. The standard ordered subset expectation maximization (OSEM) algorithm (subset 33, integration 3) was utilized for the reconstruction of PET images. To determine amyloid positivity, whole-brain visual assessment used methodology adapted from previously described techniques.5
MRI acquisition
MRI images were obtained at the MRI center of the St. Vincent Hospital using a 3T Siemens Verio machine, and 8-channel Siemens head coil. A standard high-resolution T1-weighted volumetric magnetization prepared rapid gradient echo scans (MP-RAGE) sequence was acquired in axial orientation (160 slices, 256×240, 1 mm isotropic).
Image preprocessing and statistical analysis
Reorientation of high-resolution, skull-cropped, MP-RAGE images was conducted with the AC-PC line and the medial longitudinal fissure as points of alignment. The ICBM 152 template (Montreal Neurological Institute)10 of the SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was utilized for the normalization of the acquired images. A general linear model (GLM) analysis was used for the group comparison between the FBB (+) and the FBB (−) groups while controlling for age, gender and education. The threshold was set at p<0.05 [family wise error (FWE)] to overcome errors pertaining to multiple comparisons.
RESULTS
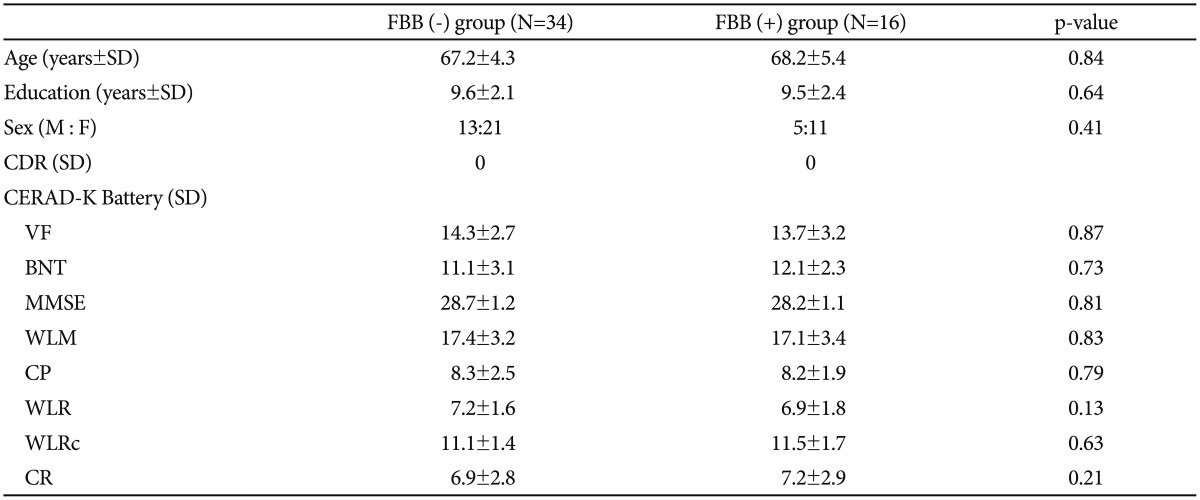
Table 1 showed the demographic data of the study participants. There was no significant differences between the FBB (+) and the FBB (−) group in demographic and the cognitive and behavioral measurements. In the group analysis, The FBB (+) group showed significantly higher amyloid retention pattern in the ACC, PRC, middle cingulate and PCC (Figure 1, Supplementary Table 1 in the online-only Data Supplement, FWE corrected p<0.05). However, these results were expanded to ACC, PRC, PCC FRC, and LT compared with the FBB (−) group when the level of significant adjusted to false discovery rate p<0.05 (Supplementary Figure 1 online-only Data Supplement). There was no significant correlation between the cognitive function scores measured by CERAD-K and the amyloid retention in the FBB (+) group. The detailed coordinates of group differences showed in the Supplemental Table 1 (online-only Data Supplement).
DISCUSSION
To our knowledge, this the first article to report results from whole brain voxel-based analysis of Aβ deposition utilizing 18F-Florbetaben on cognitively normal elderly subjects. According to our results, several notable brain regions in FBB (+) group demonstrated significantly higher Aβ retention pattern when compared with that of FBB (−) group.
Due to a short history of 18F-labeled ligands in amyloid imaging research, there have been relatively few articles on voxel-based analysis of cerebral Aβ deposition using 18F-labeled ligands. To list a few, a recent article utilizing 18F-Florbetapir discussed the potential role interaction between Aβ deposition and phosphorylated tau played on the decline of metabolism certain brain regions.11 Such interaction was most evident in the temporal cortex, frontal cortex and both cingulate cortices.11 Our results are in line with the aforementioned results, where amyloid retention was most evident the both ACC, PRC, PCC, FRC and LT. Studies on major depressive disorder patients with mild cognitive impairment (MCI) and Down syndrome and the pattern of Aβ retention in these two cohorts explored with 18F-Florbetapir yielded some meaningful results that accentuated the diagnostic utility of amyloid imaging in the prevention and early intervention of dementia.1213 As with 18F-Flutemetamol, a recent research utilized the ligand in discriminating MCI subjects into amyloid positive and negative groups, exploring possible differential factor between two groups including cortical metabolism and hippocampal volume by adopting voxel-wise surface maps.14
The brain regions with prominent Aβ deposition in FBB (+) group were regions that have been known to comprise the default mode network (DMN),15 which is frequently suggested to be areas of great connectivity where incipient Aβ deposition takes place.16 Despite the aforementioned Aβ burden in the DMN, which plays an integral role in the self-referential processing, attention and working memory,17 there was no significant correlation between the cognitive function scores and Aβ retention in our study, deviant from our original hypothesis. This phenomenon could be explained by relatively preserved brain reserve and cognitive function in this group.618 Moreover, this group of participants could be subjects with high basal cerebral metabolism that has strength to withstand the adverse effects of Aβ retention.18
Our study has several limitations that must be taken into consideration. Our sample sizes were small, making our results difficult to generalize. Moreover, extension of the study to include data on apolipoprotein E genotyping and each regional standard uptake value ratio could have added more strength in our results. Further studies including the aforementioned variables could increase validity and objectivity. Lastly, although validated to a certain level proving accuracy in detecting a Aβ deposition,19 additional standardized data on 18F-Florbetaben could be of help for more active utilization of this ligand in the clinical setting.
In summary, our study results are in line with previous findings where Aβ deposition mainly affected brain regions with active connectivity at rest. Moreover, further exploration on the relationship between brain reserve and amyloid burden will unravel underlying pathologic mechanism that precedes actual manifestation of AD symptoms.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A02036578).
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC15C1405).
References
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.4306/pi.2017.14.6.883.
Supplementary Table 1
MNI coordinates of brain regions representing results from voxel-wise analysis comparing amyloid deposition pattern between FBB (+) and FBB (−) group by using 18F-Florbetben brain PET
Supplementary Figure 1
Results from voxel-wise analysis comparing amyloid deposition pattern between FBB (+) and FBB (−) group by using 18F-Florbetben brain PET The results were color-coded by FDR-corrected T-statistic values (FDR corrected p<0.05). FBB: Florbetaben, PET: positron emission tomography, FDR: false discovery rate.