Association between Thioridazine Use and Cancer Risk in Adult Patients with Schizophrenia-A Population-Based Study
Article information
Abstract
Objective
Several cell line studies have demonstrated thioridazine’s anticancer, multidrug resistance-reversing and apoptosis-inducing properties in various tumors. We conducted this nationwide population-based study to investigate the association between thioridazine use and cancer risk among adult patients with schizophrenia.
Methods
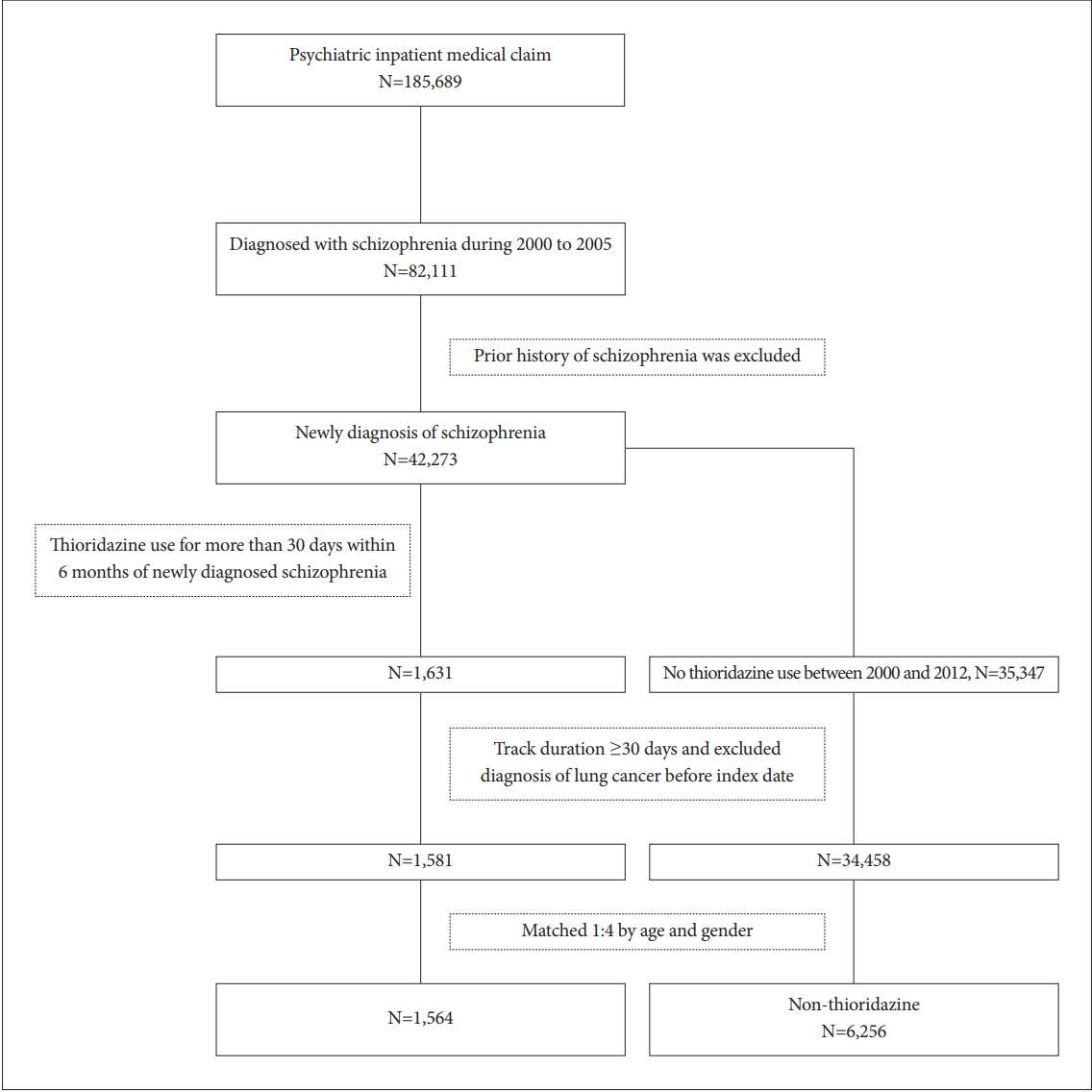
Based on the Psychiatric Inpatient Medical Claim of the National Health Insurance Research Database of Taiwan, a total of 185,689 insured psychiatric patients during 2000 to 2005 were identified. After excluding patients with prior history of schizophrenia, only 42,273 newly diagnosed patients were included. Among them, 1,631 patients ever receiving thioridazine for more than 30 days within 6 months were selected and paired with 6,256 randomly selected non-thioridazine controls. These patients were traced till 2012/12/31 to see if they have any malignancy.
Results
The incidence rates of hypertension and cerebrovascular disease were higher among cases than among matched controls. The incidence of hyperlipidemia, coronary artery disease and chronic pulmonary disease did not differ between the two groups. By using Cox proportional hazard model for cancer incidence, the crude hazard ratio was significantly higher in age, hypertension, hyperlipidemia, cerebrovascular disease, coronary artery disease and chronic pulmornary disease. However, after adjusting for other covariates, only age and hypertension remained significant. Thioridazine use in adult patients with schizophrenia had no significant association with cancer.
Conclusion
Despite our finding that thioridazine use had no prevention in cancer in adult patients with schizophrenia. Based on the biological activity, thioridazine is a potential anticancer drug and further investigation in human with cancer is warranted.
INTRODUCTION
Thioridazine, a phenothiazine derivative, is a low-potency first-generation dopamine antagonist for treating patients with schizophrenia. Several preclinical studies reported the potent treatment effect of thioridazine on various types of cancer cells [1-5]. Studies have revealed that thioridazine has antiproliferative activity and promote apoptosis in gastric cancer, cervical cancer, endometrial cancer, breast cancer, neuroblastoma, glioma, leukemia, and ovarian cancer [4-9]. These studies have demonstrated thioridazine’s anticancer, multidrug resistance-reversing and apoptosis-inducing properties in various tumor cell lines [5,10-14]. Although the mechanisms of protective effect of thioridazine are not clear, thioridazine was reported to effectively suppress tumor growth activity by targeting the PI3K/Akt/mTOR/p70S6K signaling pathway [4]. The mechanism of apoptosis induction in phenothiazine-treated leukemic cells is associated with inhibition of mitochondrial DNA polymerase and decreased ATP production, which are crucial events for the viability of cancer cell [8]. Additionally, thioridazine has been shown to sensitize drugresistance cancer cells by Pglycoprotein inhibition [15].
However, existing literature are limited to cell line studies. Previous research that investigates the anticancer effect of thioridazine by using large human sample is still lacking. In a population-based 9-year follow-up study, other than breast and cervical/uterine cancer, patients with schizophrenia have a lower risk of getting cancer (1.93%) than patients without schizophrenia (2.97%) [16]. This finding suggests that schizophrenia might be associated with a tumor suppressor gene or antipsychotic drugs that have anti-neoplastic effects, as suggested by other studies [17,18]. Because thioridazine is mainly prescribed for patients with schizophrenia, this study investigated the effect of thioridazine on the cancer risk by using a nationwide cohort of patients with schizophrenia.
METHODS
Patient selection
The study data were retrieved from the Psychiatric Inpatient Medical Claim (PIMC) dataset of the National Health Insurance Research Database (NHIRD) of Taiwan, which contains information on inpatient expenditures by admissions, ambulatory care expenditures of visits, details of ambulatory care and inpatient orders, and a registry of beneficiaries from 2000 to 2012. Caseness was operationalized as having two or more outpatient diagnoses or one inpatient diagnosis of schizophrenia, and extracted diagnostic code information from PIMC dataset according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Moreover, the data were linked to the Catastrophic Illness Claim Dataset to confirm the diagnosis. First-stage data collection of PIMC was applied for patients with schizophrenia during 2000–2005 and thioridazine was prescribed for more than 30 days within 6 months of newly diagnosis of schizophrenia. Secondly, the end of study was defined as onset of cancer, withdrawal from the social insurance, or December 31, 2012 whichever came first. These patients were followed-up from index date to 2012 end to see if they had any malignancy which includes malignant neoplasm of lip, oral cavity, and pharynx (ICD-9-CM=140–149), malignant neoplasm of digestive organs and peritoneum (ICD-9-CM=150–159), malignant neoplasm of respiratory and intrathoracic (ICD-9-CM=160–165), malignant neoplasm of bone, connective tissue, skin, and breast (ICD-9-CM= 170–176), malignant neoplasm of genitourinary organs (ICD-9-CM=179–189), malignant neoplasm of other and unspecified sites (ICD-9-CM=190–199), malignant neoplasm of lymphatic and hematopoietic tissue (ICD-9-CM=200–208). This study was approved by the Research Ethics Commitee at China Medical University Hospital, Taiwan (No. CMUH105-REC3-016).
Demographic variables including age, gender, and income were recorded at index date. Comorbid medical diseases included hypertension (ICD-9-CM=401–405), hyperlipidemia (ICD-9-CM=272.0–272.4), cerebrovascular disease (ICD-9-CM=430–438), coronary artery disease (ICD-9-CM=410–414). Since NHI files didn’t include smoking habit, we used chronic pulmonary disease (ICD-9-CM=490–496, 500–505, 506.4) as proxy for smoking status. These comorbidities were defined within one year before the index date.
Figure 1 depicted the flow chart of sample selection. For cases, we selected patients with schizophrenia received thioridazine while control patients had no thioridazine exposure during study period. During Jan. 2000 to Dec. 2005, patients diagnosed with schizophrenia (n=82,111) were included. Patients ever diagnosed with schizophrenia before index date were excluded. The index date was defined when thioridazine was prescribed for more than 30 days within 6 months of newly diagnosis of schizophrenia (n=1,631). Patients who had a prior history of cancer before thioridazine use were excluded for analysis. Follow-up duration for less than 30 days was also excluded. Controls were matched 4:1 on age and gender for each case at index date of enrollment.
Statistical analysis
We used χ2 test for categorical variables and t test for continuous variables to compare demographic and clinical characteristics between cases and controls. Cox proportional hazard model was used to estimate the adjusted hazard ratio (aHR) of cancer for thioridazine use after controlling for potential confounding factors. Confounding factors includes age, gender, and other medical illnesses. The log-rank test was used to depict the survival curves of thioridazine use versus no thioridazine use after adjusting for cofounders. The data was analyzed by SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
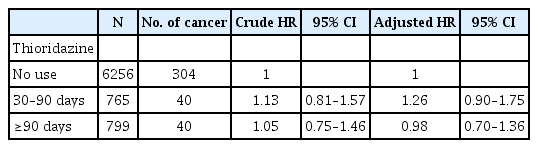
A total of 185,689 insured psychiatric patients during 2000 to 2005 were identified. After excluding patients with prior history of schizophrenia, only 42,273 newly diagnosed patients were included. These patients were traced till 2012/12/31 to see if they have any malignancy. Table 1 showed the demographic data and medical illnesses of the cases and controls. Monthly income was significantly different between patients and matched controls (p=0.005). The incidence rates of hypertension and cerebrovascular disease were higher among cases than among matched controls (p=0.005 and 0.004, respectively). The incidence of hyperlipidemia, coronary artery disease and chronic pulmonary disease did not differ between the two groups. The time to event (cancer) in patient and control groups was 5.4 and 5.5 years (p=0.784). Follow-up duration was 9.7 (SD 3.2) and 10.0 (SD 3.0) years (p<0.001), respectively. By using Cox proportional hazard model for cancer incidence, the crude HR was significantly higher in age, hypertension, hyperlipidemia, cerebrovascular disease, coronary artery disease and chronic pulmornary disease (Table 2). However, after adjusting for other covariates, age and hypertension remained significant in aHR (1.05, 95% CI 1.05–1.06; 1.41, 95% CI 1.03–1.93). Figure 2 showed the 12-year survival curve was similar between the cases and controls (p=0.504). We did a subgroup analysis to compare the effect of thioridazine exposure on cancer risk. The risk ratio of cancer incidence was not significantly different among the patients who were not prescribed thioridazine, thioridazine use for 30–90 days, or thioridazine use for more than 90 days (Table 3).
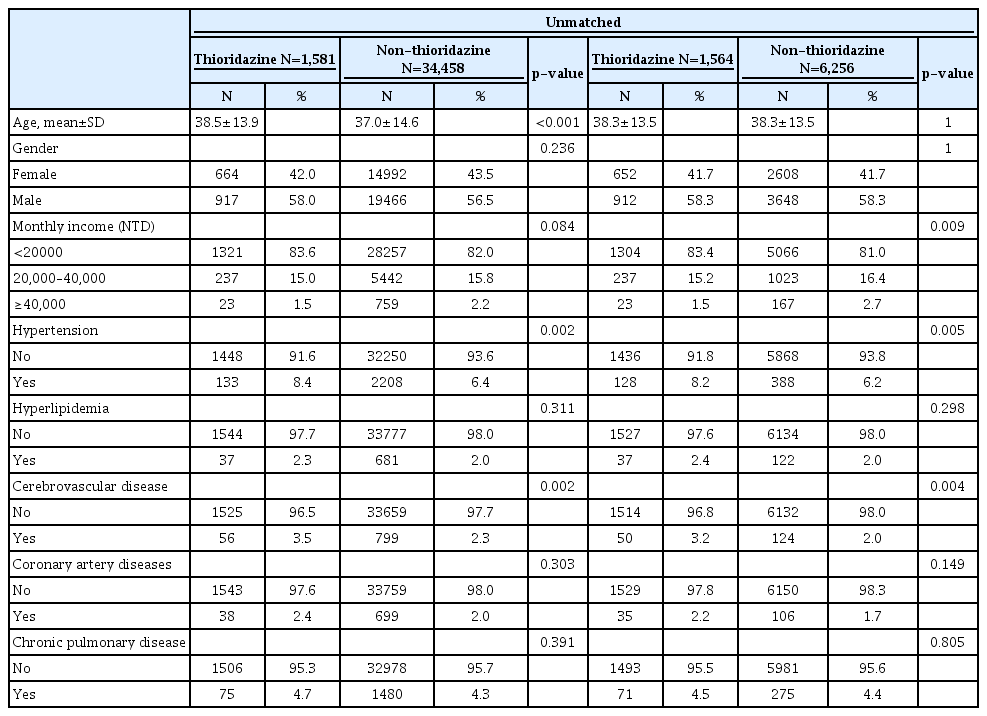
Demographic characteristics and co-morbid medical illnesses in patients with schizophrenia and matched controls

Cox proportional hazard model for cancer incidence in patients with schizophrenia and matched controls
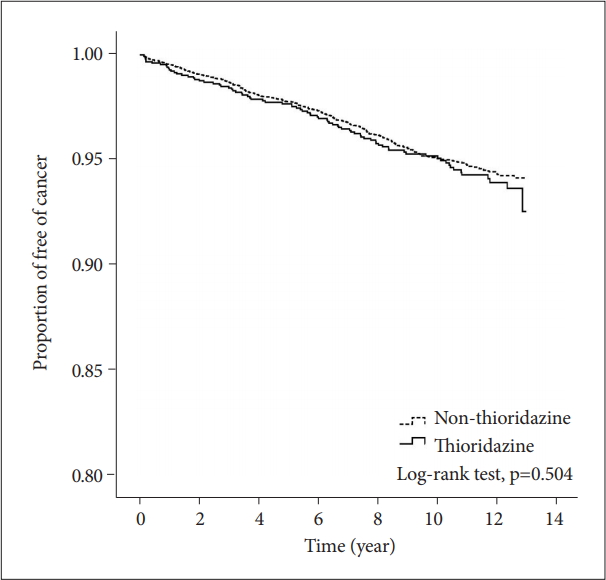
Survival analysis of cancer incidence in patients with schizophrenia receiving thioridazine and matched controls.
DISCUSSION
To our knowledge, this is the first study that investigated the use of thioridazine and incidence of cancer among patients with schizophrenia by using nationwide data. The findings revealed that age and hypertension were highly correlated with cancer risk. Thioridazine use for at least 30 days had no significant association with cancer in adult patients with schizophrenia. This is contrary to with other cell line studies demonstrating that thioridazine had antitumor properties [5,10-14].
Hypertension was highly correlated with cancer risk in our study, however, observational studies have shown inconsistent results for the association between blood pressure and cancer risk. This discrepancy might be due to study method, patient selection or definition of blood pressure. Stocks et al. [19] suggested a small increased cancer risk overall in men with elevated blood pressure level and a higher risk for cancer death in men and women. Radisauskas et al. [20] reviewed the epidemiological evidence to overview the association between blood pressure, serum lipids and cancer risk. They found arterial hypertension is closely linked with renal cell cancer development, independently of sex. Studies suggest that a higher total serum cholesterol level is linked with higher risk of colorectum, colon, prostate and testicular cancer and lower risk of stomach, liver and hematopoietic and lymphoid tissues cancer. There was also a positive association between serum triglycerides and esophageal, colorectal, lung, renal, thyroid cancer [20]. However, in our study, after controlling for other factors, only comorbid hypertension instead of hyperlipidemia, cardiovascular disease or coronary artery disease was highly correlated with cancer. The diagnosis of any other co-morbid conditions was completely dependent on ICD codes, the severity of each illness cannot be retrieved via NHIRD which may be related to cancer development. In addition to hypertension per se, antihypertensive drugs may play a role in regard to cancer incidence. Use of antihypertensive medications for more than 5 years, when compared with no use, was associated with a modest increased risk of invasive breast cancer [21]. Aging was highly correlated with the cancer incidence. Cancer can be considered an age-related disease because the incidence of most cancers increases with age. Some of the same biologic mechanisms that regulate aging also may be involved in the pathogenesis of age-related diseases such as cancer [22].
We had several explanations why thioridazine use had no association with cancer in our study. First, the anti-proliferative activity of thioridazine takes place at high concentrations to ensure intercalation of the compound between nucleic bases [4]. Thioridazine could dramatically inhibit sphere formation of lung cancer stem cells [12] and elicited cytotoxic effects on the gastric cancer cell lines [5] in a dose-dependent manner. However, thioridazine was mainly prescribed as a low-potency antipsychotic drug in clinical practice, its use was limited due to multiple side effects, for example, somnolence, constipation, dry mouth, dose-related risk of QT prolongation and retinitis pigmentosa. The usual recommended dose of thioridazine is 200 to 800 mg per day which may not be sufficient for anti-proliferative activity. Second, several studies showed thioridazine alone is not effective in treatment of certain cancers [11,23]. Meng et al. [11] found the apoptosis of endometrial cancer cells was upregulated after treating with thioridazine and medroxyprogesterone. They suggested thioridazine could promote medroxyprogesterone to inhibit the growth of cells in endometrial cancer. Also, thioridazine exhibited a synergistic effect to carboplatin and doxorubicin in inducing cancer cell apoptosis [23,24]. This might suggest us that thioridazine had no preventive but therapeutic effect in cancer treatment. Third, follow-up duration between thioridazine and non-thioridazine group was significantly different (9.7 years vs. 10.0 years, p<0.001). Exposure duration of thioridazine was more than 30 days within 6 months of newly diagnosed schizophrenia, this might not be long enough to prevent cancer occurrence. Fourth, previous studies were mainly focused on cell lines, the anticancer effect of thioridazine may not apply to human bodies.
Our study had some limitations. First, the causal relationship cannot be inferred from our cross-sectional study. Second, patients with schizophrenia are often lacking insight and their medication compliance is compromised. It is not easy to determine whether thioridazine prescription is commensurate with thioridazine utilization. Third, the NHRID files did not include records of other potential confounding factors, for example, severity of psychiatric and comorbid medical illnesses which may be related to cancer. Fourth, follow-up period (12 years) may not be long enough to detect the difference of cancer risk, and the average age of patients was not old enough (38 years old) to develop cancer.
Albeit our finding that thioridazine use for more than 30 days had no significant association with cancer, based on the biological activity of thioridazine, such as interference with membrane function, DNA repair, signaling pathways, and apoptosis induction, renders thioridazine a potential anticancer drug and further investigation concerning thioridazine use in human with cancer is warranted.
Acknowledgements
The authors are grateful to Taiwan’s National Health Research Institutes for providing the NHIRD. This study was supported by Research Ethics Committee at China Medical University Hospital, Taiwan (No. CMUH105- REC3-016).