The Impact of the COVID-19 Pandemic and Social Distancing on Cognition of Alzheimer’s Disease Patients
Article information
Abstract
Objective
The risk of rapid cognitive decline in Alzheimer’s disease (AD) during the coronavirus disease 2019 (COVID-19) pandemic has been recognized. The purpose of this study was to investigate the cognitive decline in such patients during the COVID-19 pandemic by evaluating changes in their cognitive measure parameters before and after the pandemic.
Methods
This was a retrospective cohort study conducted in AD patients during their first visit and one-year regular follow-up for testing cognitive function at the Geriatric Psychiatry Clinic of Seoul St. Mary’s Hospital. Changes in the Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR), and Sum of Box for CDR (CDR-SB) scores were investigated. A time series analysis was performed to determine whether there was a significant difference in the MMSE, CDR, and CDR-SB scores of AD patients in pre- and post–COVID-19 periods.
Results
Overall, 130 AD patients aged 60 to 93 years were assessed. Their baseline mean MMSE score was 22.30 which had decreased to 21.08 at the one-year follow-up. Before November 2019, the average CDR differences for one year was 0.06, but after November 2019, it increased to 0.36 (p<0.001). Before November 2019, the average of the CDR-SB change value for one year was 1.69, but after November 2019, it increased to 3.00 (p<0.001). The difference in MMSE values for one year was not statistically significant. The time series analysis revealed a significant increase in the CDR and CDR-SB scores by approximately 0.47 (p=0.005) and 2.39 (p=0.002), before and after November 1, 2019, respectively.
Conclusion
This study revealed that the COVID-19 pandemic and social distancing worsen cognitive function in AD patients rapidly. Exposure to the COVID-19 pandemic and social distancing for at least seven months worsen cognitive decline significantly. Therefore, in order to minimize the adverse effects of the cognitive decline in these patients, the period of social distancing should be minimized.
INTRODUCTION
The World Health Organization (WHO) declared the novel coronavirus disease 2019 (COVID-19) outbreak a global pandemic on March 11, 2020 [1]. Consequently, many countries implemented social distancing or lockdowns to prevent the spread of several acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although social distancing or lockdowns are critical measures of reducing the spreads of COVID-19, they may have a negative effect on the physical or mental health of the elderly.
The global prevalence of Alzheimer’s disease (AD) is rapidly increasing. Currently, AD cannot be cured with existing treatment options; however, the deterioration of cognitive function or alleviation of symptoms may be possible using these options. It is well known that cognitive, social, and physical stimulation as well as drug treatment play an important role in delaying the deterioration of cognitive function in the elderly with AD [2-5]. Based on these studies, many countries have started multimodal stimulation programs for elderly population [4]. They were able to engage in various activities such as exercise, music, art, studying, game, and social activities prior to the COVID-19 pandemic. As public welfare facilities for the elderly or gyms were closed, any formal and informal cognitive stimulation programs have been stopped during pandemic. Especially AD patients are exposed to ‘de-stimulation environment’ of staying at home, further reducing opportunities for physical activities, social interaction, and cognitive stimulation [6].
More objective data are required to provide a basis for policymakers when making decisions regarding the welfare of the elderly, in order to improve methods to efficiently and effectively control the novel infectious disease pandemic while simultaneously minimizing the detrimental effects of social distancing. Therefore, the purpose of this study was to investigate the indirect effects of COVID-19 pandemic and social distancing on cognitive decline in AD patients, by investigation of changes in their cognitive measure parameters before and after the COVID-19 pandemic.
METHODS
This was a retrospective cohort study of AD patients regularly followed-up for cognition at the Geriatric Psychiatry Clinic of the Psychiatry Department of The Catholic University of Korea, Seoul St. Mary’s Hospital, which is a tertiary referral university hospital.
Study definitions
On March 22, 2020, the Head of the Central Disaster and Safety Countermeasures Headquarters in South Korea urged the public to refrain from going out, and announced intensive social distancing measure to restrict the facilities with a high risk of group infection which included religious and public welfare facilities. Therefore, the date of enforcement of social distancing due to the COVID-19 pandemic was defined as March 22, 2020 in this study. Social distancing measures were implanted for approximately two years and a month and were lifted from April 18, 2022, in South Korea.
Study population
This study included outpatients who were first diagnosed with AD with annual testing for cognitive function at the Geriatric Psychiatry Clinic of the Psychiatry Department at Seoul St. Mary’s Hospital from January 2019 to December 2020 were enrolled in the study. The patients infected with COVID-19 were excluded because COVID-19 infection can directly affect cognitive function. Patients suffering from cognitive impairment underwent a detailed clinical interview, standardized neuropsychological test battery, namely the Korean version of the Consortium to Establish a Registry for Alzheimer’s disease (CERAD-K), laboratory test, apolipoprotein E genotyping, and brain magnetic resonance imaging (MRI). We used criteria of the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) [7] and National Institute on Aging and Alzheimer’s Association for a diagnosis of AD [8].
Cognitive function test
The following cognitive function test parameters were measured on the day of the first visit and on annual follow-up: Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR), and Sum of Box for CDR (CDR-SB). MMSE is a screening tool developed to simply evaluate the overall cognitive function of the elderly within a short period. It includes tests of orientation, attention, memory, language, and visual-spatial skills. The total score of the MMSE is 30, and lower score correspondences to lower cognitive function. The CDR is the most widely used tool to assess the severity of dementia. The six domains are evaluated, including memory, orientation, judgement and problem-solving, community affairs, home and hobbies, and personal care. The score for each domain can range from 0 to 3 points (0, 0.5, 1, 2, 3). The doctor conducts a detailed interview with the patient and caregiver to determine the functions of the six domains. The CDR score is calculated in two ways; the first method determines the total CDR as 0, 0.5, 1, 2, and 3 based on the memory score, and the second method is a CDR-SB, which sums the scores of all six domains. A higher CDR or CDR-SB score corresponds to increased severity of dementia.
Statistical analysis
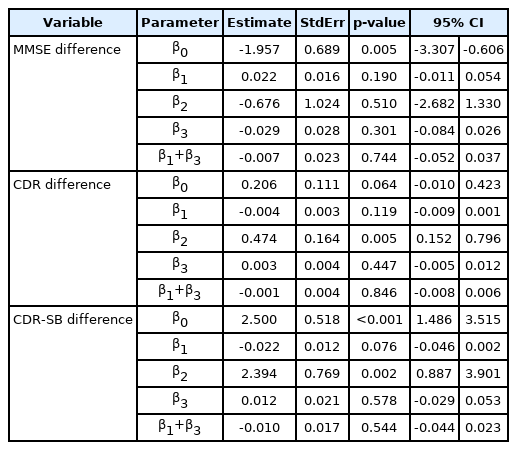
We used the SAS® software, version 9.4. (SAS Institute Inc., Cary, NC, USA) for data analysis. Independent t-test or Wilcoxon’s rank sum test was performed for the continuous variables, while chi-square test or Fisher’s exact test was performed for the categorical variables. An interrupted time series (ITS) analysis was applied to determine the effect (level and trend changes) of social distancing measures on cognitive deterioration of AD patients. We analyzed the level or trend changes for differences in the MMSE, CDR, and CDR-SB scores, before and after social distancing. The ITS is best understood as a simple but powerful tool used for evaluating the impact of a policy change or quality improvement program on the rate of an outcome in a defined population of individuals. A time series—repeated observations of a particular event collected over time—is divided into 2 segments ideally. The first segment comprises rates of the event before the social distancing, and the second segment is the rates following social distancing. “Segmented regression” is used to measure statistically the changes in level (immediate) and trend (slope) after the social distancing period compared to before the social distancing period [9]. The model is: y=α+β1T+β2X+β3XT+ε. y=outcome variable, α=intercept, β=coefficients, T=time (1, 2, 3, …, N), X=study phase (0 during pre-interruption and 1 during post-interruption), XT=time after interruption (0 during pre-interruption and 1, 2, 3, …, n during post-interruption), ε=error.
y is the difference between baseline and after one year of MMSE, CDR, or CDR-SB scores, β1 is the baseline trend of cognitive function before the social distancing, β2 is the level change in cognitive function immediately after the social distancing, and β3 is the slope change, which is mean rate of change in cognitive function after the social distancing.
Ethics approval
The Institutional Review Board of the Catholic Medical Center approved this study protocol (KC21WISI0678).
RESULTS
Baseline characteristics of the study populations
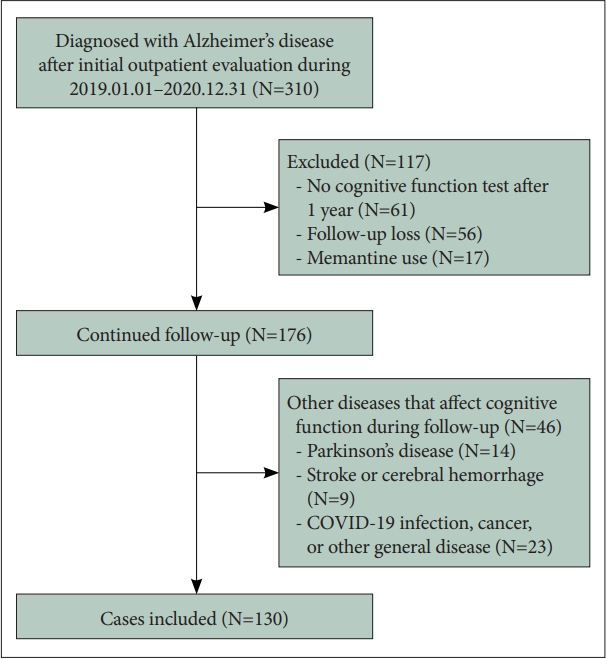
A total of 310 patients were first diagnosed with AD at the Geriatric Psychiatry Clinic from January 2019 to December 2020. One-hundred thirty patients qualified for inclusion in the analysis after excluding patients who did not undergo cognitive function test after 1 year, who did not follow up at the clinic, and those who were diagnosed with other diseases affecting cognitive function during the follow-up period (Figure 1).
The median age at the first visit-diagnosed AD in the study was 80 years, and included 34.62% which were male. The median body mass index (BMI) were 23.05 kg/m2. The median years of education was 12 years. Twenty-five patients had a family history of dementia whereas 105 did not. The number of patients with diabetes, hypertension, and hyperlipidemia were 28, 73, and 34, respectively. The patients with apolipoprotein E4 genotype were 34.62%. There were 127 patients on cholinesterase inhibitors therapy (Table 1).
Changes in cognitive functions
Changes in cognitive function over one year in AD patients before and after the social distancing due to COVID-19 pandemic were compared using Wilcoxon signed rank test or Wilcoxon rank sum test (Table 2). As a result of exploring the time of the first visit at which a significant change in difference of annual cognitive function occurred with the Wilcoxon rank sum test and time series analysis, it was confirmed that the change in difference of annual cognitive function occurred from November 2019 (first visit time, baseline time). When comparing patients diagnosed with AD after November 2019 with those before, there were no statistically significant differences in baseline clinical characteristics (Table 2). Before November 2019, the average CDR differences for one year was 0.06, but after November 2019, it increased to 0.36 (p<0.001). Before November 2019, the average of the CDR-SB change value for one year was 1.69, but after November 2019, it increased to 3.00 (p<0.001). The difference in MMSE values for one year was not statistically significant.

Comparison of the scores of cognitive function test before and after the social distancing due to COVID-19 pandemic
Through time series analysis, the level change of the difference in CDR and CDR-SB values for one year was observed from patients who received their first visit in November 2019. In contrast, the slope was mostly stable (Table 3, Figure 2). Before November of 2019, CDR differences between baseline and 1 year after were about 0.21. CDR differences between baseline and 1 year after were increasing by about 0.47 between the before and after November of 2019 (p=0.005). Before November of 2019, CDR-SB differences between baseline and 1 year after were about 2.50. CDR-SB differences between baseline and 1 year after were increasing by about 2.39 between the before and after November of 2019 (p=0.002).
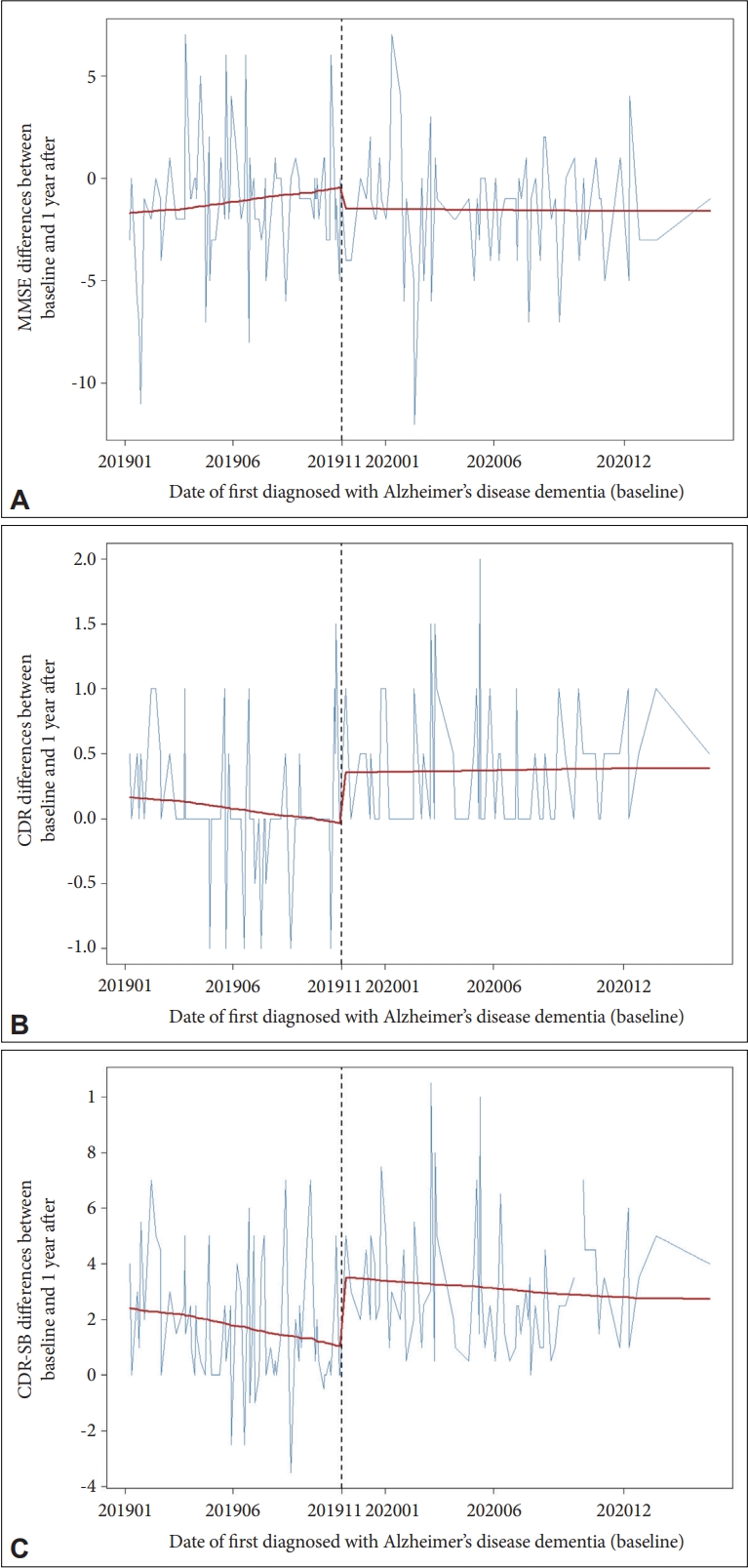
Changes in cognitive function over one year in Alzheimer’s disease patients before and after the social distancing due to COVID-19 pandemic. Change in difference of annual cognitive function occurred from November 2019. The vertical dashed line indicates when the COVID-19 pandemic and social distancing started to have an effect. A: MMSE differences between dates of first diagnosed Alzheimer’s disease (baseline) and 1 year after. B: CDR differences between baseline and 1 year after. C: CDR-SB differences between baseline and 1 year after. MMSE, Mini-Mental State Examination; CDR, Clinical Dementia Rating; CDR-SB, Sum of Box for CDR.
DISCUSSION
Over the past two years, cases of the COVID-19 have occurred continuously around the world, with the COVID-19 pandemic still ongoing. There is a lack of data on the harmful effects of the COVID-19 pandemic and social distancing on the cognition of elderly. This study was conducted to shed light on the detrimental effects of the COVID-19 pandemic and social distancing on the cognition of AD patients. We investigated whether there was a significant difference in the MMSE, CDR, and CDR-SB scores of AD patients in pre- and post–COVID-19 periods. This study revealed that the COVID-19 pandemic and social distancing worsen cognitive function in AD patients rapidly. Exposure to the COVID-19 pandemic and social distancing for at least seven months worsen cognitive decline significantly.
Recently, several studies have been reported on the direct association between the COVID-19 infection and psychiatric disorders. Taquet et al. [10] reported that, a diagnosis of the COVID-19 was associated with increased incidence of a first psychiatric diagnosis in the following 14 to 90 days compared with other six other health events such as influenza, other respiratory tract infections, skin infection, cholelithiasis, urolithiasis, and fracture of a large bone. In this study, 1.6% (95% CI, 1.2–2.1) people older than 65 years were diagnosed with psychiatric disorders in the 14–90 days following the COVID-19 diagnosis [10]. Furthermore, the COVID-19 infection was associated with increased mortality in schizophrenia [11], mood disorder [12], and dementia [13]. The indirect effect of the COVID-19 pandemic, such as social isolation was reported in dementia patients from different countries. The most consistent finding was a higher rate of behavioral and neuropsychiatric symptoms due to social isolation in dementia patients [6,14]. The most recent study conducted in Italy reported that social isolation in the COVID-19 pandemic caused a significant worsening of cognitive decline in people with dementia [15]. This study compared MMSE score differences per year in three different years (2018, 2019, 2020) and revealed that in the 2020-group, affected by the COVID-19 pandemic, significant worsening of MMSE score was seen compared to earlier periods. Our study compared MMSE, CDR, and CDR-SB score differences per year in the pre–COVID-19 period and the post–COVID-19 period and revealed that in the patients affected by the COVID-19 pandemic and social distancing, significant worsening of CDR and CDR-SB score was seen compared to pre–COVID-19 periods. However, the difference in MMSE score was not statistically significant. In other words, it was confirmed that the severity of overall cognitive function including memory, orientation, judgement and problem-solving, community affairs, home and hobbies, and personal care was more affected by the COVID-19 pandemic and social distancing than global mental status measured by the MMSE. While the study conducted in Italy targeted all dementia patients, our study only targeted AD patients. In addition, our study used the difference between the cognitive function values at the time of initial diagnosis and the test values one year after. Furthermore, we excluded the patients infected with the COVID-19. In this way, we tried to minimize the influence of factors affecting cognitive deterioration other than the COVID-19 pandemic and social distancing.
Multiple factors may be associated with an increase of post-pandemic cognitive decline. The social distancing measure have urged the society to avoid get-togethers and going to public places. Furthermore, welfare facilities closure results in the stoppage of welfare facilities-based multimodal cognitive and physical stimulation programs. These two combined measures have placed dementia patients in a situation where the majority do not meet the daily recommended physical and social activity. In particular, since most of the social activities of dementia patients are performed in welfare facilities, the decrease in social and interpersonal activities due to social distancing might be greater in this population. Indeed, social distancing due to the COVID-19 pandemic involves different social, emotional, psychological, and physical modifications, each one with a potential contribution to increase distress. Decrease in physical activities cause muscle loss, which is also known to be related to cognitive decline [16]. The pandemic in itself can contribute to trigger fear and contagion phobia. There is also increase evidence that stress-related psychological symptoms can contribute to cognitive decline and increase risk of AD [17]. In patients with dementia, social distancing induced of new behavioral and psychological symptoms, with additional stress-related symptoms for caregivers [6]. Depression or anxiety in caregivers might further worsen patients’ behavioral and psychological symptoms, eventually, leading to cognitive decline in dementia patients.
In our study, the duration of exposure to the COVID-19 pandemic and social distancing was found to be a significant factor for rapid cognitive decline. The level change in difference of annual cognitive function (CDR and CDR-SB scores) occurred from November 2019. Patients diagnosed with AD in November 2019 were impacted by social distancing from April to October 2020. In other words, it can be seen that exposure to the COVID-19 pandemic and social distancing for at least 7 months affects faster deterioration of cognitive function. Therefore, stopping social distancing at the earliest should be a priority for policymakers. Should circumstances following the COVID-19 pandemic regard it inevitable to keep social distancing, interventions should be made to increase opportunities for physical and social activity for elderly.
We reported a few limitations to this study. First, since the present study was conducted at a tertiary referral hospital, the AD patient included were more likely to have caregivers who were more pay attention to the patient’s cognitive status. Therefore, the changes in cognition during social distancing might have been underestimated, when compared to the estimate in the general population. Second, because the situation of each case was not considered as a factor affecting cognitive decline during social distancing, caution needs to be taken when applying the study results to general population. Third, this study did not assess the behavioral or psychiatric symptoms such as depressive symptoms, anxiety, or sleep problems which might affect the severity of cognitive function. Fourth, there are no objective functional assessment data such as activities of daily living of subjects. Furthermore, we did not assess whether patients were managing their underlying disease, such as taking medication or visiting the hospital regularly. Nevertheless, the most important strength of this study is that this is the first study finding the effects of social distancing and the COVID-19 pandemic on cognitive decline of AD patients.
To conclude, this study revealed that the COVID-19 pandemic and social distancing induced a more rapid worsening of cognitive function, especially severity of general cognition in AD patients. Furthermore, exposure to the COVID-19 pandemic and social distancing for at least seven months had a negative effect on cognitive decline. Rapid worsening of the severity of cognition in AD patients after social distancing due to the COVID-19 pandemic is a serious concern. The policymakers have to be interested in the increased severity of cognition in AD patients following social distancing during the COVID-19 pandemic. In order to minimize the adverse effects of the cognitive decline that social distancing has on AD patients, the policymakers should minimize the period of social distancing or provide a way to maintain physical and cognitive activities during period of social distancing.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
Chang Uk Lee, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Chang Uk Lee, Soo Hyun Joo. Data curation: Soo Hyun Joo. Formal analysis: Soo Hyun Joo. Methodology: Chang Tae Hahn, Soo Hyun Joo. Sofware: Soo Hyun Joo. Supervision: Chang Uk Lee. Validation: Chang Tae Hahn. Visualization: Soo Hyun Joo. Writing—original draf: Soo Hyun Joo. Writing—review & editing: Chang Uk Lee, Chang Tae Hahn.
Funding Statement
None
Acknowledgements
We thank to member of Medical Excellence for analyzing data.