Genotyping Sleep Disorders Patients
Article information
Abstract
Objective
The genetic susceptibility factors underlying sleep disorders might help us predict prognoses and responses to treatment. Several candidate polymorphisms for sleep disorders have been proposed, but there has as yet inadequate replication or validation that the candidates may be useful in the clinical setting.
Methods
To assess the validity of several candidate associations, we obtained saliva deoxyribonucleic acid (DNA) samples and clinical information from 360 consenting research participants who were undergoing clinical polysomnograms. Ten single nucleotide polymorphisms (SNPs) were genotyped. These were thought to be related to depression, circadian sleep disorders, sleep apnea, restless legs syndrome (RLS), excessive sleepiness, or to slow waves in sleep.
Results
With multivariate generalized linear models, the association of TEF rs738499 with depressive symptoms was confirmed. Equivocal statistical evidence of association of rs1801260 (the C3111T SNP in the CLOCK gene) with morningness/eveningness and an association of Apolipoprotein E (APOE) rs429358 with the Epworth Sleepiness Scale (ESS) were obtained, but these associations were not strong enough to be of clinical value by themselves. Predicted association of SNPs with sleep apnea, RLS, and slow wave sleep were not confirmed.
Conclusion
The SNPs tested would not, by themselves, be of use for clinical genotyping in a sleep clinic.
Introduction
In a sleep clinic, the causes of our patients' distress can be complex and obscure. We might better understand prognosis and likely responses to treatment interventions when the etiologies can be identified. For example, a patient with excessive sleepiness may have both sleep apnea and depressive symptoms, both of which may have genetic components. A patient complaining of insomnia may have genetically-mediated delayed sleep phase disorder and restless legs combined with a cognitive-behavioral disturbance. Since reported sleep duration appears to be as much as 50% heritable, both short sleep complaints and long sleep complaints may arise in large part from genetic susceptibilities.1-4 Recognizing genetic susceptibilities for depression, delayed sleep phase, restless legs or short sleep, for example, might help confirm diagnostic formulations and anticipate the responses to treatment options.
With the rapid pace of genomic discoveries, many research groups and not a few corporations are exploring the idea that genotyping individuals may help guide their health care. With the long-term goal of applying genotyping to our care of sleep clinic patients, we have begun to genotype patient volunteers on a research basis when they are undergoing clinical polysomnograms. We hoped to validate the association of candidate sleep disorder susceptibility factors with various symptom phenotypes, and to assess the extent to which genotypes might become helpful in identifying the etiologies of our patients' complaints.
To date, only a limited number of genetic polymorphisms have been suggested as causes of the diverse sleep disorders. The alleles of 4 single nucleotide polymorphisms (SNPs) possibly related to circadian rhythms and affective disorders, 3 SNPs possibly related to sleep apnea, 2 SNPs possibly related to restless legs syndrome (RLS) or periodic limb movements in sleep (PLMS), and 1 SNP possibly related to slow wave sleep were identified (Table 1). These 10 SNPs were selected from the larger number with previously-reported sleep associations based on ease of genotyping and the predicted likelihood of association in our Clinic sample.
Our prospective hypotheses were as follows. Based on a finding among sib pairs with a proband with early-onset recurrent unipolar major depression,5 it was prospectively predicted that the common T allele of rs738499 of TEF (a gene participating in circadian system feedback loops) would be associated with the QIDS-SR measure of depressed mood. Likewise, based on prior evidence that rs2314339 (a SNP in NR1D1 or Rev-erb-alpha, a component of circadian system feedback loops) was associated with bipolar disorder, it was predicted that rs2314339 would be associated with the QIDS-SR, even though rs2314339 had not been significantly associated with unipolar major depression in the prior study.5 Because of the evident functional role of circadian SNPs, it was also prospectively predicted that the BALM morningness-eveningness scale would be associated with rs738499, rs2314339, the Phe876Leu amino-acid changing SNP in PER26 and rs1801260 (the C3111T SNP in the CLOCK gene previously reported to be related to morningness-eveningness and to insomnia).7 Further, based on previous reports, it was prospectively predicted that sleep apnea might be associated with rs405509 and rs429358 [SNPs related to the Apolipoprotein E 4 (APOE 4) genotype] and rs1800629, a -308 tumor necrotic factor α (TNFα) promoter polymorphism which influences TNFα expression.8-13 Also, rs5751876 in ADORA2A, the A2A adenosine receptor, is associated with higher electroencephalography (EEG) amplitudes especially below 5 Hz regardless of sleep or wake, so we predicted rs5751876 would be associated with the slow wave sleep percentage or with total sleep time (TST).14 We further predicted that MEIS1 rs12469063 and MAP2K5 rs 1026732 would be associated with restless legs frequency, the 4 essential questions for diagnosis of RLS, and the polysomnographic periodic limb movement index.15-17 Several secondary hypotheses were also examined.
Methods
Consent and questionnaire
When a clinical research staff member was available and the clinical context permitted, patients arriving at our sleep laboratory for clinical recordings or who had recently undergone sleep recordings were invited to participate in our genetics research program. First, the study was explained. Then a signed consent approved by the Scripps Institutional Review Board was obtained. If it had not already been completed as part of our clinical patient intake procedure, a comprehensive sleep questionnaire was completed (form can be requested from the authors). The questionnaire asked about habitual bedtimes, falling-asleep times, wake-up times, and uptimes both on weekdays and weekends. It included dozens of multiple-choice questions about the frequency and severity of various sleep symptoms. Embedded in the questionnaire were the BALM morningness-eveningness scale (a simplified-language abbreviation of the Horne-Östberg morningness-eveningness scale18 with which it correlates r=0.96), the QIDS-SR (a modern self-report depression rating scale which has been validated against the Hamilton Depression Rating Scale and against DSM-IV major depression diagnoses),19,20 and the Epworth Sleepiness Scale (ESS) (a widely used rating of daytime sleepiness),21 along with the four questions essential for a diagnosis of RLS, often associated with PLMS.22-24
Polysomnogram
Participants underwent standard clinical polysomnograms in our sleep laboratory using Compumedics Profusion PSG 2.10.0.131 software on a Compumedics E Series platform. Recordings were scored according to the current criteria of the American Academy of Sleep Medicine. In 59% of the recordings, the patient was awakened after sleep apnea was detected, and nasal continuous positive airway pressure (CPAP treatment) was titrated during the latter part of the recording. In these split night studies, scoring of the polysomnograms was divided into two portions. Phenotypes were computed from the first portion of split recordings unmasked by CPAP, except in 10 recordings where there was so little sleep in the first portion that use of the CPAP portion appeared more representative.
DNA collection
The participants were asked to provide about 2 cc of saliva in an Oragene deoxyribonucleic acid (DNA) Self-collection Kit (DNA Genotek, Inc., Ottawa, Ontario, Canada), used according to the manufacturer's directions. DNA was stored with preservative in the Oragene kits at room temperature until it could be extracted and purified according to the manufacturer's protocol, producing an average of 80-120 mcg. of purified DNA. DNA was then frozen in split samples for further study.
Phenotype determination
In accordance with the consent granted, polysomnographic data, clinical diagnoses from multiple visits, and clinical referrals were compiled with coded identifiers in research data bases. For this study, the following 10 quantitative phenotypic traits were derived from the data bases: 1) ESS score for daytime sleepiness,21 2) BALM score for the morningness-eveningness circadian trait,18 3) QIDS-SR score for current depression,19,20 4) number of 4 essential RLS complaints (restless legs essential features) answered affirmative,23 5) answer on a 6-point scale of frequency of "a feeling of restless legs", 6) polysomnographic TST in minutes, 7) polysomnographic slow wave sleep percentage, i.e., stage 3%+stage 4%, 8) polysomnographic periodic leg movement index, 9) polysomnographic apnea-hypopnea index (AHI), 10) a global score summarizing the extent of clinician documentation of an obstructive sleep apnea diagnosis. This apnea diagnostic score was compiled from arbitrary weights for the number of visits and interval of months over which an apnea diagnosis was recorded clinically; likewise the number of visits, interval, and stated confidence in which a separate research diagnosis of obstructive apnea was recorded; whether a referral for CPAP or mandibular advancement device treatment was recorded; the polysomnographic AHI (if 15 or more) and the index of oximetry desaturations of ≥4% (if 15 or more per hour). Each of these phenotypic traits reflecting a manifestation of sleep disorders could then be examined for its association with particular genotypes.
Assays
DNA was genotyped for the first 360 participants at the DNA Core Laboratory of the Molecular and Experimental Medicine division of the Scripps Research Institute, using allele-specific oligonucleotide hybridization.25 A single subject was dropped for inadequate DNA quantity. Technical difficulties assaying certain SNPs and expense limited this report to 10 SNPs from a much larger number which might have been of interest (Table 1).
Statistics
The quantitative phenotypes were tested as dependent variables in multivariate generalized linear models, using as independent predictors the participant's age (continuous), gender (binary), race (self-reported and recoded as white or non-white), and the genotypes. Additive, dominant, and recessive models were tested in these analyses when applicable. For the dominant and recessive models, each SNP was modeled as a dichotomous variable with one genotype (either dominant or recessive) chosen as the reference group, and the other two genotypes combined into one category. For the additive model, the number of dominant alleles was used as the variable. No adjustments for multiple comparisons were conducted in the analyses, since the primary analyses tested independent prospective hypotheses. Analyses were implemented in Systat V.12 (Systat Software, Inc., Chicago, IL, USA).
Results
The 359 participants ranged from age 21 to 92 years, with a mean age of 57.4 (±standard deviation 13.9) years. Participants were 33% women and 67% men. The sample was 90% White by self-report. The global obstructive apnea scores ranged from 3 to 94 with a mean of 25. Apnea global scores indicated some clinical consideration of an apnea diagnosis for every patient, though in many cases, a clinician might have coded an apnea diagnosis based on clinical supposition which later was not confirmed by polysomnography. The number of times clinical and research diagnoses of apnea were recorded, treatment referrals, AHIs, and oxygen desaturation indices (ODIs) (4% ODIs) contributed 48%, 15%, 10%, 17%, and 10% respectively to the global apnea scores. In 10% of participants, sleep apnea (any kind) was not a first diagnosis, e.g., primary diagnoses of insomnia, delayed sleep phase disorder, rapid eye movement behavior disorder, and narcolepsy. Polysomnographically, 22% had an AHI of less than 5 (suggesting no clinically significant sleep apnea), and only 47% had an AHI of greater than 15 (a commonly-used threshold indicating when CPAP treatment is indicated.) Parenthetically, 43% had ODI<5. In only 10 of the recordings was it necessary to utilize the CPAP titration portion of a split PSG for analysis because there was insufficient sleep in the first part. Of these 10 recordings during CPAP, 7 had an AHI<5, but presumably the CPAP suppressed the AHI as compared to what would be observed without CPAP. Partly because of the split night procedures, the mean TST for the recording segments utilized was only 253 minutes.
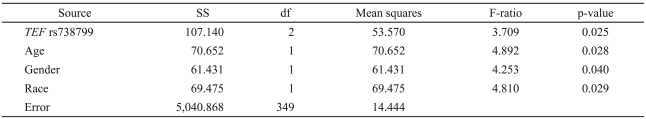
Examining the association of the QIDS-SR depression score with TEF rs738499 as well as age, gender, and race as independent control variables, the overall regression yielded R2=0.06, with all independent variables being significant (Table 2). The adjusted mean QIDS-SR was 6.5±standard error (SE) 0.9 for the GG rs738499 genotype, 6.9±SE 0.4 for the GT genotype, and 7.9±SE 0.3 for the TT genotype. Controlling for age, gender, and race, the rs738499 T allele being present or absent was significantly associated with the QIDS-SR score with R2=0.03 and p=0.025, whereas the significance was p=0.008 for an additive model for the T allele, e.g., TT>TG>GG, where T is the more common allele. Since this association had been prospectively predicted as the highest priority hypothesis, this association was considered significant. The NR1D1 SNP genotype was not significantly related to the QIDS-SR.
Although with CLOCK rs1801260, the BALM score averaged 33.8±SE 1.9 in the CC genotypes, compared to 36.8±SE 0.8 in the TC genotypes and 37.4±SE 0.7 in the TT genotypes, the probability of association was only p=0.071, two-tailed. This result might be considered equivocally significant by one-tailed criteria, considering that the association and its direction (less morningness with the C allele) was predicted in advance based on prior work.7 The NR1D1, TEF, and PER2 SNP genotypes were not significantly associated with the BALM score.
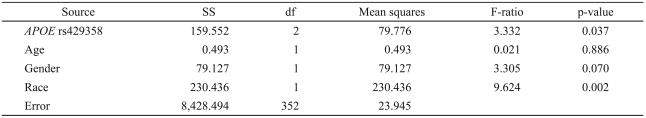
The predictions that TNFα rs1800629 and the APOE SNPs rs405509 and rs429358 would be associated with the AHI or with the global apnea score were not confirmed. However, in rs429358, the more common T allele was weakly associated with the ESS (Table 3), an association which had not been prospectively predicted. The other two SNPs, rs405509 and rs1800629, were not associated with the ESS.
MEIS1 rs12469063 and MAP2K5 rs1026732 were not significantly associated with the four diagnostic questions for RLS syndrome, with the reported frequency of "restless legs," or with the polysomnographic periodic leg movement index. ADORA2A rs5751876 was not significantly associated with polysomnographic TST nor with the slow wave sleep percentage.
In retrospective Post Hoc computations (which do not meet Bonferroni significance criteria for multiple testing), the T allele of TEF rs738499 was associated with the percentage of slow wave sleep (sleep stages 3 and 4), controlling for covariates of gender, age, and race (p<0.05). Similarly, EEG TST was associated with the C allele of CLOCK rs1801260 (p=0.04) and the A allele of TNFα rs1800629 (p<0.025). Selecting on those roughly 50% of participants with AHI<15, to minimize confounding with apnea, the PLMI was greater among those with the C allele of CLOCK rs1801260 (p<0.05), the number of the 4 RLS questions answered affirmatively was associated both with the A allele of MEIS1 rs12469063 (p<0.01, but not the predicted direction of association) and with the T allele of TEF rs738499 (p<0.025), and the T allele of NR1D1 rs2314339 was positively associated with the reported frequency of a feeling of restless legs. No such associations were noted in the group with AHI≥15. Moreover, no such associations were noted when groups answering the four RLS questions were dichotomized into those answer yes to all 4 or <4.
Discussion
The genomic revolution is a work in progress, providing remarkable developments in methodology, exciting new discoveries, and many disappointments and frustrations. For example, the whole genome association chips have led to numerous new discoveries but also to an appreciation that tens of thousands of subjects may be needed to locate the relevant polymorphisms for complex disorders such as hypertension and diabetes, in which dozens or hundreds of variants may make small contributions to the phenotype.26-28 Few of the associations demonstrated by this new technology explain more than a small fraction of each disease phenotype,29 perhaps because many rare variants rather than a few common variants produce the genetic susceptibility. Distinguished observers have argued that there is little value for individual patients in current genotyping methods for most major disorders.27
Research in a patient sample undergoing clinical polysomnograms had the strength of relevance to patients for whom diagnostic determination and treatment choice was a high priority. Another advantage was clinical support for the diagnostic assessment. Serious limitations of this approach included lack of consistent diagnostic criteria, since the national standards changed during the study, diagnostic uncertainty or multiple diagnoses in many instances, and the variability in the portion of the polysomnogram which could be utilized if split-night CPAP titration procedures were begun. Also, even without CPAP titrations, there is considerable night-to-night variability in polysomnographic measures such as the periodic leg movement index.30 With a larger patient sample, we might have been able to demonstrate more significant associations of genotypes with sleep phenotypes, but the lower the attributable risk of an association and the larger the sample needed to detect association with a genotype, the less the clinical value of genotype association will be. In the prospectively predicted results, none of the associations were strong enough to be considered statistically conclusive without further replication. Of the post-hoc analyses, none of the nominal findings would have been significant after correction for multiple testing, so they should merely be considered hypothetical relationships needing further replication.
Because depression is commonly comorbid with sleep apnea, insomnia, restless legs, and narcolepsy, depression is very commonly seen among Sleep Clinic patients. A large component of depression is hereditary,31 so depression might be one of the sleep-disturbing disorders most identifiable by genetic means. Unfortunately, the genetic basis of depression has proved surprisingly elusive, as indicated by the disappointing results of the GAIN whole genome association study, which used over 3,500 subjects.32 Our result replicating an association of TEF rs738799 with a quantitative trait of depression was therefore of considerable scientific interest, though the level of statistical confidence even combining this and the earlier study5 was not so great as to make further replication unnecessary. It is possible that rs738799 is not the functional SNP associated with depression, in which case, a search for the functional SNP or a group of functionally significant polymorphisms might yield an association of greater magnitude. By itself, rs738799 might not display sufficient association with depression to make its identification valuable for predicting the usefulness of antidepressant treatment.
Remarkable new findings with RLS and PLMS may promise that genotyping could identify a larger proportion of the risk for these disorders than most whole genome association results for other disorders.15,33,34 To our surprise, we were not able to confirm that genotyping identified a useful proportion of the risk for restless legs or PLMS, but we were only able to genotype two of the several SNPs for which the strongest associations had been reported, and MEIS1 rs12469063 was technically difficult to genotype in our assay.15,33 Part of the explanation for our failure to demonstrate association may be the limited positive predictive value of the four criterion questions which we used to identify restless legs.23,35 Another problem may be the interference of sleep apnea with recognizing RLS, since there was a nominally significant association of the RLS questions with MEIS1 rs12469063 among those participants with lower AHI scores. The retrospective evidence for nominal association with PLMI or restless legs symptoms with three circadian SNPs (CLOCK rs1801260, TEF rs738499, and NR1D1 rs2314339), consistent with the strong circadian modulation of restless legs symptoms, might be explained by regulation of dopamine by MAOA, which is under control of the circadian network.36,37 Circadian system genetic feedback loops regulate the expression and activity of the CLOCK-ARNTL heterodimer, which binds to an e-box promoter site on the MAOA gene. The greater the expression of MAOA, the faster the degredation of dopamine, thus regulating dopaminergic mediation of restless legs symptoms.
There is as yet little knowledge of the genetic factors in sleep apnea. We could not confirm reports that TNFα and APOE are substantial risk factors for sleep apnea, as some reports have suggested,8-13 but our results support the recent meta-analysis which concluded that APOE is not associated with sleep apnea.38 The SNP rs405509 does not fully define the APOE4 haplotype, but it is very highly associated with Alzheimer's Disease.39 As we expand our sample, we will have more participants with no indication of sleep apnea, which may make the discrimination of sleep apnea syndrome stronger. However, since half of the participants had an AHI<15, the sample may have been well-balanced for detecting polymorphisms associated with apnea in need of treatment. We found statistically equivocal evidence that an APOE variant is associated with sleepiness, but the possible association noted was too weak to be of any current practical value. Likewise, we have as yet little knowledge of the genetic factors determining sleep duration, despite its considerable heritability.1-4
In ADORA2, rs5751876 had been reported to influence EEG slow wave amplitude,14 but it had not been reported to influence either TST or the slow wave sleep percentage. We tested the novel hypotheses that a polymorphism which increases EEG slow waves should increase the percentage of slow wave sleep scored and perhaps the TST, but could not confirm these hypotheses. The nominal associations of TEF rs738499 with slow wave sleep percentage and of CLOCK rs1801260 and TNFα rs1800629 with TST should not be regarded as conclusive findings unless they can be replicated. Patel et al.40 found that TNFα concentrations were higher in the blood of those reporting longer habitual sleep, but TNFα was associated with shorter objective PSG durations.
Examination of four SNPs of the circadian system provided an equivocal confirmation of the influence of rs1801260 in CLOCK, which did not quite satisfy the strictest significance criteria. This previously-reported association has been controversial,7,41-45 but the current results tend to support a weak association. Linkage disequilibrium extends throughout the large CLOCK gene, so this SNP may be linked to the true functional SNP only in certain populations. Elsewhere, we have reported SNPs with a stronger association with the BALM scale.5 We believe that as knowledge of circadian polymorphisms grows, it may be possible to better recognize the genetic circadian component of sleep disorders.
This study replicated some reported associations of SNP polymorphisms with sleep phenotypes, found other reported associations not to be replicable, and contributed preliminary suggestions of some new associations. These results add to the body of knowledge of genetic polymorphisms which influence sleep. Significance of associations of some of these 10 SNPs with phenotypes might be more conclusively demonstrated with larger samples, e.g., the association of the CLOCK SNP rs1801260 with the BALM, but if more subjects are needed, such associations are likely to explain too little phenotypic variance to be of clinical value by themselves. Our sample was larger than that of most of the papers which originally reported the associations examined. Because so many associations with phenotypes in genetics have proven nonreplicable,46,47 failures to replicate associations are informative, as a minimum for highlighting the modesty of effect sizes.
The discovery of the genetic factors in sleep disorders is still in its infancy. New reports of polymorphisms associated with sleep disorders are appearing. For example, our assays were performed before the very recent report relating polymorphisms in a T cell receptor to narcolepsy,34 and there are many other interesting SNPs we have not yet examined. As the knowledge base expands, many more polymorphisms worth testing will emerge, and more extensive genotyping will become progressively more economically practical as the technology develops. In conditions with complex genetic susceptibility factors, it may become possible to identify a useful amount of genetic susceptibility by assaying much larger numbers of markers. The advancement of genetic knowledge to assist sleep disorders treatment depends on the foresighted collection of still larger DNA samples of several homogeneous racial groups, along with reliable data bases of phenotypes. We remain hopeful that future discoveries will make genotyping useful for the sleep clinician.
Acknowledgments
J. Steven Poceta, MD, Diem Vo, Susan Abel, RN, and Richard Loving, RN, DNSc recruited participants for this research. Pauline Lee, Ph.D. and Jessica Nichols genotyped the Sleep Clinic participants in the Core DNA Laboratory, which was supported by the Stein Endowment Fund at the MEM division of the Scripps Research Institute. The research was supported by Scripps Clinic Academic Funds.