|
 |
- Search
| Psychiatry Investig > Volume 11(3); 2014 > Article |
Abstract
Objective
The present study aimed to investigate whether graded doses of Bacopa Monniera (BM) extract could produce antidepressant-like effects in chronic unpredictable stress (CUS) induced depression in rats and its possible mechanism(s).
Methods
Rats were subjected to an experimental setting of CUS. The effect of BM extract treatment in CUS-induced depression was examined using behavioral tests including the sucrose consumption, open field test and shuttle box escape test. The mechanism underlying the antidepressant-like action of BM extract was examined by measuring brain-derived neurotrophic factor (BDNF) protein and mRNA expression in brain tissues of CUS-exposed rats.
Results
Exposure to CUS for 4 weeks caused depression-like behavior in rats, as indicated by significant decreases in sucrose consumption, locomotor activity and escape latency. In addition, it was found that BDNF protein and mRNA levels in the hippocampus and frontal cortex were lower in CUS-treated rats, as compared to controls. Daily administration of the graded doses of BM extract during the 4-week period of CUS significantly suppressed behavioral changes and attenuated the CUS-induced decrease in BDNF protein and mRNA levels in the hippocampus and frontal cortex.
Depression is a commonly occurring, debilitating, and life threatening psychiatric disorder characterized by a pervasive low mood, loss of interest or pleasure in daily activities and suicidal tendencies.1,2 Recent studies reported that depression and anxiety may occur together in association with sub-threshold level of depression and anxiety. Anxiety may also predispose depression or vice-versa or symptoms of anxiety and depression may be external manifestation of underlying cause. Other symptoms, such as sleep and psychomotor disturbances, suicidal tendencies, decreased food-intake and body-weight are also often present.3,4
Brain derived neurotrophic factor (BDNF) is a member of the nerve growth factor family.5 It is essential for growth, maintenance, cellular differentiation and survival of neurons in the central nervous system. It acts through its axon specific receptor tyrosine kinase (Trk). Postmortem analyses have revealed lower levels of BDNF in patients with major depression,6 while BDNF infusion into the brain has been found to produce antidepressant like action.7 Clinical studies have found decreased BDNF levels in the blood of depressive patients,8,9,10 while antidepressant treatment seems to be able to normalize BDNF levels.11,12 BDNF is thus an attractive candidate for current research in the pathophysiology of depression and the molecular mechanism of action of antidepressants.13,14,15,16
Herbal products have recently become the drugs of choice and investigated for the search of novel and better tolerated molecules from plant sources.17 Therefore, it is desirable to seek new antidepressants drug through herbal treatment.
Bacopa monniera (BM) (L.) Wettst (Brahmi, family: Scrophulariaceae), is commonly mentioned as a rasayana in Ayurvedic materia medica and is advocated to be useful in clinical mental disorders, anxiety disorders, obsessive compulsive disorders, hysteria, epilepsy and insomnia.18 Several clinical studies have confirmed the beneficial actions of BM and its pharmacological actions are mainly attributed to the bacosides present in it.19,20 According to pharmacological profile of BM, it is reasonable to assume that the extract may have some neuroprotective activities. Based on these merits, the present study aimed to evaluate the standardized extract of BM against CUS-induced behavioral, biochemical, and neuropathological alterations in rats.
We used chronic unpredictable stress (CUS) induced depression as a model to determine whether chronic BM treatment can alleviate stress-induced behavioral abnormalities and corresponding changes in HPA axis. In addition, to investigate the possible molecular mechanisms mediating the therapeutic effects of BM, we investigated the expression levels of BDNF protein and mRNA in the hippocampus and frontal cortex of rat brain.
In this study, the antidepressant like effect of extract of BM (40, 80, and 120 mg/kg body weight) was evaluated in a rat model of depression induced by CUS. The molecular mechanism underlying the antidepressant like action of BM extract was investigated by measuring BDNF protein and mRNA levels in brain tissues of CUS exposed rats. This preclinical study confirmed 80 mg/kg dose as the most effective dose of BM to reverse the effects of CUS on behavior, BDNF protein and mRNA expressions.
We purchased extract of BM (≥40% w/w) from Natural Remedies Pvt. Ltd., Bangalore, India. The extract contains total bacosides 40.0-50.0%, along with a number of chemical constituents, namely bacoside A3 (>2.7%), bacopaside II (>3.6%), Jujubogenin isomer of bacopa saponin C (>4.5%), bacopa saponin C (>3.0%), bacopaside I (>4.5%). This was prepared by dissolving 450 mg of dried powder in 80 mL distilled water and administered intragastrically once daily.
Imipramine hydrochloride (IMI), a tricyclic antidepressant, was purchased from Sigma-Aldrich (St. Louis, MO) and used as positive control for antidepressant action.
Male Sprague-Dawley rats weighing 180-220 g were obtained from the animal care and maintenance division of Chittaranjan National Cancer Institute (CNCI), Kolkata, India. The animals were maintained on a 12 h light/dark cycle (lights on at 6:00 a.m., lights off at 6:00 p.m.) under controlled temperature conditions (22±2℃), and given standard food and water ad libitum. They were allowed to acclimatize for 7 days before use. All experiments conformed to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA, New Delhi). The experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) of Raja Peary Mohan College, Uttarpara, Hooghly (W.B.). Effort was made to minimize the number and suffering of the animals.
The rats were randomly assigned to six groups of eight subjects: Control, CUS plus vehicle, CUS plus BM (40 mg/kg), CUS plus BM (80 mg/kg), CUS plus BM (120 mg/kg), and CUS plus IMI (20 mg/kg). BM and IMI were each given intragastrically 30 min before each stressor applied once every day for 4 weeks. BM and IMI were dissolved in normal physiological saline (0.9% NaCl). BM and IMI were dissolved in physiological saline, and the animals in the control and CUS groups were treated with equal amounts of physiological saline (10 mL/kg) as vehicle. The CUS procedure was performed as described by Mao et al.21 with a slight modification. Briefly, CUS consisted of a variety of unpredictable stressors, including 48 h food deprivation, 24 h water deprivation, 5 min cold water swim (at 4℃), 1 min tail pinch (1 cm from the end of the tail), foot shock (1 mA, 1s duration, average 1 shock/min) for 60 min and overnight illumination. One of these stressors (in random order) was given every day between 9:00 a.m. and 12:00 a.m. for 4 weeks. Control (unstressed) animals were undisturbed except for necessary procedures such as routine cage cleaning. On day 28, behavior of the stressed rats was observed and the rats were sacrificed to assess any biochemical, neurochemical changes that may have occurred and to measure gene expression levels in the hippocampus and frontal cortex of rat brain.
The sucrose preference test was carried out at the beginning and the end of 4 week period of CUS exposure. The test was performed as described previously,22 with minor modifications. Briefly, before the test, the rats were trained to adapt to sucrose solution (1%, w/v): two bottles of sucrose solution were placed in each cage for 24 h, and then one bottle of sucrose solution was replaced with water for 24 h. After the adaptation procedure, the rats were deprived of water and food for 24 h. The sucrose preference test was conducted at 9:00 a.m. The rats were housed in individual cages and given free access to two bottles containing 100 mL of sucrose solution (1%, w/v) and 100 mL of water, respectively. After 60 min, the volume of both the consumed sucrose solution and water was recorded.
The open field test was carried out at the beginning and the end of 4 week period of CUS exposure. The test was modified from previously described methods.23 Briefly, the open field apparatus consisted of a square wooden arena (100×100×50 cm) with a black surface covering the inside walls. The floor of the wooden arena was divided equally into 25 squares marked by black lines. In each test, a single rat was placed in the center of the arena and allowed to explore freely. The number of crossings (squares crossed with all paws) and rearings (raising of the front paws) were recorded during a test period of 5 min. This apparatus was cleaned with a detergent and dried after occupancy by each rat.
After 4 weeks of CUS procedure following BM or IMI treatment, rats were subjected to shuttle box testing. The escape avoidance test was carried out in a two-way shuttle box (60×20×20 cm) with plexiglass walls fitted with a floor consisting of stainless steel rods separated by 1.0 cm. The floor was divided into two equal size chambers by a wood partition (1.5 cm above the grid floor). Subjects were placed in the shuttle-box, allowed to habituate to the test environment for 3 min, and then were subjected to 30 avoidance trials (30-s inter-trial interval). The number of escape failures, defined as the absence of a crossing response before or during shock delivery, was recorded.
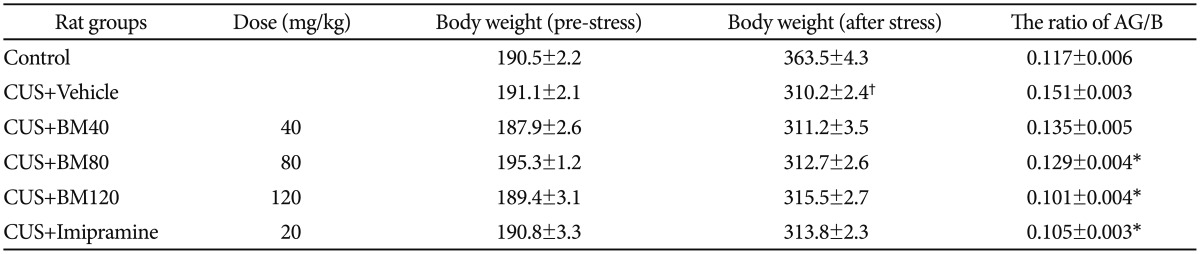
Body weight (gm.) of all rats were measured categorically pre-stress and post stress sessions. Adrenal gland weights (mg) of all rats were evaluated through adrenalectomy after the completion of 4 weeks CUS procedure.
Twenty four hours after the completion of the last behavioral test, the rats were sacrificed by decapitation. The whole brain of each rat was rapidly removed and chilled in an icecold saline solution. Various brain areas, including the hippocampus and frontal cortex, were dissected on a cold plate and immediately frozen in liquid nitrogen. The tissue samples were stored at -80℃ until assay.
50-100 mg tissue of each subject from hippocampus and frontal cortex was extracted using RIPA buffer [20 mM Tris-HCL (pH 8), 150 mM NaCl, 1 mM EDTA, 50 mM NaF, 1 mM Na2MoO4, 0.5 mM Na3VO4, 5 mM Na2P2O7, 1% Triton X-100, 0.5% Na deoxycholate, 0.1% SDS, 10% glycerol, 10 µg/mL leupeptin, 10 µg/mL aprotinin, 0.01 mM phenylmethyl sulfonyl fluoride (PMSF), 1 mg/mL pepstatin A, and 10 mM benzamidine]. Supernatant was prepared by centrifugation (REMI, India) at 15,000 rpm for 10 min at 4℃. Protein content was determined by the Bradford method (Bio-Rad, CA, USA).
The content of BDNF protein was measured using a commercially available enzyme linked immunosorbent assay (ELISA) kit (Chemicon International, Temecula, CA, USA) according to the manufacturer's instructions. The hippocampus and frontal cortex samples were weighed and homogenized in 10 fold volume of lysis buffer (RIPA buffer). The homogenate was centrifuged at 14,000×g for 30 min at 4℃, and the supernatants were collected and stored at -80℃ until assay. All samples and standards were applied in duplicate into 96 well immunoplates precoated with mouse antihuman BDNF antibody, which were incubated on a shaker overnight at 4℃. After washing four times, biotinylated mouse antihuman BDNF antibody was added and the immunoplates were incubated for 3 h at room temperature. After washing, streptavidin HRP conjugate solution was added and the immunoplates were incubated at room temperature for 1 h. TMB/E substrate was added and the immunoplates were incubated at room temperature for 15 min. The plates were immediately read by a microplate reader at 450 nm (Systronics, India). The detection limit of the assay is 7.8 pg/mL.
TrkB was immunolabeled with the Western blot technique as described below. Equal volumes of soluble fractions containing 60 µg of protein from both hippocampus and pre-frontal cortex were electrophoresed on 15% (weight-volume ratio) polyacrylamide gel (Mini-PROTEAN® Tetra Cell with Mini-Trans Blot®, Bio-Rad, USA). The blots were incubated overnight at 4℃ with primary anti-TrkB polyclonal antibodies (Chemicon, USA), (1:500 dilution in 3% BSA). After stripping the membrane with stripping solution, Anti-β-actin monoclonal antibody (1:10000 dilution in 3% BSA, Sigma, USA) was used as a housekeeping protein to reduce internal blot variability.
Nitrocellulose membranes were washed three times in TBST and incubated with HRP-conjugated anti-sheep IgG (1:1000) for 2 hours at room temperature. Immuno-reactive bands were visualized using the Enhanced chemiluminescence (ECL) (Santa Cruz, USA). Before starting the experiment, the dilution of each antibody and the duration of the exposure of the nitrocellulose membranes on autoradiographic film were standardized. The bands on the autoradiograms were quantified as mentioned above and the optical density value (OD) of each band was analyzed with the electrophoresis image analysis system (Smartview 2001, S/N: SV-0002202, Japan). The optical density of each protein was corrected by using the optical density of the corresponding β-actin band. The values are presented as a percent of the control.
Total RNA from the hippocampus and frontal cortex was isolated with RNASure® Mini Kit according to the manufacturer's protocol. Concentrations of extracted RNA were calculated from the absorbance at 260 nm. The quality of RNA was assessed by absorbances at 260 and 280 nm, with a ratio of A260 to A280 ranging from 1.9 to 2.1 considered acceptable. Total RNA (1.5 mg) was transcribed using a high capacity cDNA reverse transcription kit (The Maxima® First Strand cDNA Synthesis Kit) according to the manufacturer's protocol. Assays on demand primers for BDNF (Rn02531967 s1) and beta-actin (Rn00667869 m1) were purchased from Applied Biosystems, Inc. Realtime Quantitative polymerase chain reaction (PCR) analysis was performed with a Maxima® SYBR Green qPCR Master Mix (2X), using the StepOnePlus™ realtime PCR system (Applied Biosystems, Inc., Foster City, CA). Sequence Detection System Software (Version 1.0, Applied Biosystems, Inc., Foster City, CA) was used for data analysis. The relative expression of BDNF mRNA was normalized to the amount of β-actin in the same cDNA.
The Statistical Package for the Social Science (SPSS) 15.0 was utilized for statistical analyses.
All data were expressed as mean±SEM and have been statistically analyzed with ANOVA followed by Student's t-test. p values less than 0.001 and 0.05 were considered statistically significant. p value means the probability value and SEM means standard error of the mean.
Figure 1 shows the effect of BM treatment on the sucrose consumption in CUS exposed rats. Before CUS treatment, there was no significant difference in the percentage of sucrose consumption among all rats (data not shown). A 4 week period of CUS exposure along with graded doses of BM treatment exhibited significant variation (F2, 39=110.14; p<0.05). Chronic CUS exposure resulted in a significant reduction (tCUS=25.33, df=19, †p<0.05) in the sucrose consumption level in the animals, as compared to the normal controls (i.e. non CUS exposed rats). Chronic treatment with BM at daily doses of 80 and 120 mg/kg resulted in significant increases in the sucrose consumption in CUS exposed rats (tBM80=13.62, df=19, *p<0.05 and tBM120=19.21, df=19, *p<0.05 respectively) (Figure 1), as compared to only CUS exposed stressed rats. Treatment with IMI (20 mg/kg), a positive control, also significantly increased the level of sucrose consumption in CUS exposed rats (tImi=19.62, df=19, *p<0.05) (Figure 1). Obtained results from this study clearly illustrated that chronic treatment with BM at daily doses of 80 and 120 mg/kg resulted in significant increase (tBM80=8.35, df=19, *p<0.05 and tBM120=12.86, df=19, *p<0.05 respectively) (Figure 1) in the sucrose consumption in CUS exposed rats than treated with BM at daily dose of 40 mg/kg.
There was no significant (tBM120=0.95, df=19, p>0.05) (Figure 1) alteration in sucrose consumption rate among BM 80 & BM 120 treated rat group rats.
Figures 2 and 3 show the effect of BM treatment on locomotor activity in CUS exposed rats. Significant variation in locomotor activity was assessed by the number of crossings (CrossingF5, 78=238.14; p<0.05) (Figure 2) and rearings (RearingF5, 78=90.51; p<0.05) (Figure 3) in the open field test. Before CUS treatment was given, there was no significant difference in the number of crossings and rearings among all rats (data not shown). A 4 week period of CUS exposure resulted in a significant reduction in the number of crossings (tCUS= 22.75, df=19, †p<0.05) (Figure 2) and rearings (tCUS=28.24, df=19, †p<0.05) (Figure 3) in rats as compared to the normal controls. Chronic treatment with graded doses of BM significantly increased the number of crossings (tBM80=11.80, df=19, *p<0.05 and tBM120=13.65, df=19, *p<0.05 respectively) and rearings (tBM80=16.15, df=19, *p<0.05 and tBM120=13.10, df=19, *p<0.05 respectively) (Figure 3) in CUS exposed rats, as compared to the only stressed rats. Long term treatment with BM at daily dose of 80 and 120 mg/kg also significantly increased the number of rearings (tBM80=6.12, df=19, *p<0.05 and tBM120=6.08, df=19, *p<0.05 respectively) (Figure 3) and crossings (tBM80=8.43, df=19, *p<0.05 and tBM120=4.69, df=19, *p<0.05 respectively) (Figure 2) in CUS exposed rats, as compared to the BM40 treated rat group. Treatment with IMI (20 mg/kg) also significantly increased the number of crossings (tImi=17.72, df=19, *p<0.05) (Figure 2) and rearings (tImi=18.91, df=19, *p<0.05) (Figure 3) in CUS exposed rats. There was no significant alteration among BM80 & BM120 treated rats in the number of crossing (tBM120=0.32, df=19, p>0.05) (Figure 2) and rearing (tBM120=1.72, df=19, p>0.05) (Figure 3).
Figure 4 shows the effect of BM treatment on learning activity in CUS exposed rats altered markedly (F5, 78=19.31; p<0.05). Learning activity was assessed by the latency period in the shuttle box escape test. Before CUS treatment was given, there was no significant difference in the escape latency among all rats (data not shown). A 4 week period of CUS exposure resulted in a significant enhancement of escape latency period (tCUS=10.48, df=23, †p<0.05) (Figure 4) in rats as compared to the controls. Chronic treatment with graded doses of BM significantly decreased the escape latency periods (tBM80=7.99, df=23, *p<0.05 and tBM120=9.6, df=23, *p<0.05 respectively) (Figure 4) in CUS exposed rats, as compared to the stressed rats. Long term treatment with BM at daily dose of 80 and 120 mg/kg also significantly reduced the escape latencies in CUS exposed rats (tBM80=3.04, df=23, *p<0.05 and tBM120=3.44, df=23, *p<0.05 respectively) (Figure 4), as compared to the BM40 treated rat group. Treatment with IMI (20 mg/kg) also significantly reduced the latency period (tImi=7.27, df=23, *p<0.05) (Figure 4) in CUS exposed rats. There was no significant alteration among BM80 & BM120 treated rats in their escape latency (tBM120=0.32, df=19, p>0.05) (Figure 4).
The effects of chronic unpredictable stress and the administration of BM extract on body weight, the ratio of adrenal gland weight to body weight (AG/B) are summarized in Table 1. No difference in the initial body weight was observed in any experimental group (data not shown). Chronic stress significantly decreased body weight and significantly increased AG/B ratio relative to the non-stressed control rats (data shown in Table 1).
Chronic stress-induced decrease in body weight was not significantly affected by the 40, 80 or 120 mg/kg BM treatment; treatment; however, the increases in the AG/B ratios were ameliorated by the drug treatment. The positive control treatment with IMI (20 mg/kg) had similar effects on the AG/B ratio (data shown in Table 1).
Figure 5 shows the significant effect of BM treatment on BDNF protein levels in the hippocampus (F5, 78=21.86; p<0.05) and frontal cortex (F5, 78=35.54; p<0.05) of CUS exposed rats. CUS significantly decreased BDNF protein levels in the hippocampus (tCUS=8.23, df=9, †p<0.001) (Figure 5) and frontal cortex (tCUS=15.09, df=9, †p<0.001) (Figure 5) of rats, as compared to the controls. BM treatment at daily doses of 80 and 120 mg/kg significantly increased BDNF protein levels in both the hippocampus (tBM80=8.65, df=9, *p<0.001 and tBM120=9.0, df=9, *p<0.001 respectively) (Figure 5) and frontal cortex (tBM80=9.21, df=9, *p<0.001 and tBM120=13.8, df=9, *p<0.001 respectively) (Figure 5) compared to CUS exposed rats. IMI treatment (20 mg/kg) also significantly increased BDNF protein levels in both the hippocampus and frontal cortex regions of CUS exposed rats (tImi=15.57, df=9, *p<0.001 and tImi=12.53, df=9, *p<0.001 respectively) (Figure 5). Recent study also illustrated that there were no significant alterations in BDNF protein levels among BM80 and BM120 treated rat groups in both hippocampus (tBM120=0.55, df=9, p>0.05) (Figure 5) and frontal cortex (tBM120=0.55, df=9, p>0.05) (Figure 5).
Long term CUS significantly reduced the expressions of BDNF cognate receptor TrkB in both the hippocampus as well as frontal cortex. The molecular weight of TrkB and β-actin were 145 kDa and 42 kDa respectively. The expression levels of TrkB, against the internal control β-actin protein level to determine their actual expression through Western Blot analysis. Long term exposure to CUS followed by administration of graded doses of BM significantly altered TrkB expression profiles in both hippocampus (F5, 78=157.62; p<0.05) (Figure 6) and PFC (F5, 78=143.54; p<0.05) (Figure 7) regions.
In the Western Blot analysis the expression profiles of TrkB in both hippocampus (tTrk B=21.62; df=9; †p<0.001) (Figure 6) and PFC (tTrk B=18.93; df=9; †p<0.001) (Figure 7) region were significantly decreased among the CUS group rats compared to untreated control group rats.
Administration of graded doses of BM significantly enhanced the expressions of TrkB. In the Western Blot analysis the expression profiles of TrkB significantly increased in hippocampus among the recovery group rats treated with BM 80 and BM 120 doses compared to CUS induced rats (tBM80=19.88, df=9, *p<0.001 and tBM120=20.89, df=9, *p<0.001 respectively) (Figure 6). Similar phenomenon was also observed in the region of PFC (tBM80=39.98, df=9, *p<0.001 and tBM120=40.23, df=9, *p<0.001 respectively) (Figure 7) too.
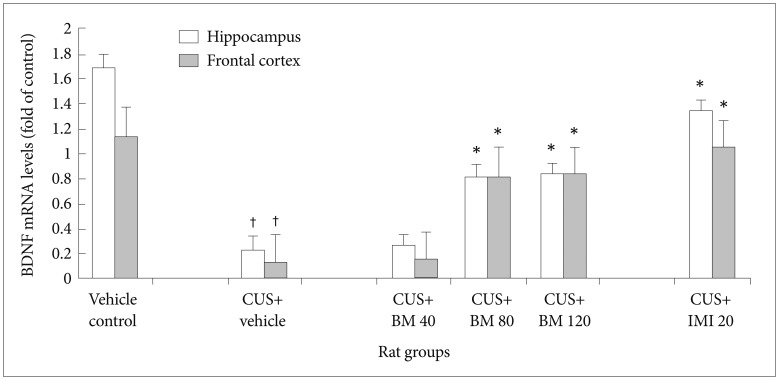
Figure 8 shows the significant effect of BM treatment on BDNF mRNA levels in the hippocampus (F5, 78=7.86; *p<0.05) and frontal cortex (F5, 78=6.96; *p<0.05) of CUS exposed rats. CUS significantly decreased BDNF mRNA levels in the hippocampus (tCUS=10.46, df=9, †p<0.001) (Figure 8) and frontal cortex (tCUS=43.96, df=9, †p<0.001) (Figure 8) of rats, as compared to the controls. BM treatment at daily doses of 80 and 120 mg/kg significantly increased BDNF mRNA levels in both the hippocampus (tBM80=15.55, df=9, *p<0.001 and tBM120=26.31, df=9, *p<0.001 respectively) (Figure 8) and frontal cortex (tBM80=17.31, df=9, *p<0.001 and tBM120=20.34, df=9, *p<0.001 respectively) (Figure 8) of CUS exposed rats, as compared to the only stressed rats. IMI treatment (20 mg/kg) also significantly increased BDNF protein levels in both the hippocampus and frontal cortex of CUS exposed rats (tImi=9.81, df=9, *p<0.001 and tImi=20.54, df=9, *p<0.001 respectively) (Figure 8). Recent study also illustrated that there were no significant alterations in BDNF protein levels among BM80 and BM120 treated rat groups in both hippocampus (tBM120=0.38, df=9, p>0.001) (Figure 8) and frontal cortex (tBM120=0.49, df=9, p>0.001) (Figure 8).
The present results showed that animals subjected to a chronic unpredictable stress regime for 28 consecutive days failed to acquire normal sucrose consumption, locomotor activity and escape response. However, sucrose consumption, locomotor activity and escape deficits could be reversed if animals were either chronically administered BM (80-120 mg/kg) or imipramine (20 mg/kg) during the stressor period. These doses have the capability to restore animal's sucrose consumption, locomotor activity, escape deficits, BDNF levels and mRNA levels in hippocampus and frontal cortex. The effects of BM were similar to those of imipramine, which was used as a positive control. The effects of chronic stress on body weight were not ameliorated by BM extract and imipramine administration.
In the present study, we investigated the effects of BM, an Indian herb, on rats exposed to CUS, using the sucrose preference, open field tests and shuttle-box test to assess their behavior, and measuring BDNF protein and mRNA levels in the hippocampus and frontal cortex. The results demonstrated that extract of BM treatment at a daily dose of 80 or 120 mg/kg not only had a potent antidepressant like effect on CUS induced depression model in rats but also attenuated the decrease in BDNF protein and mRNA levels in the hippocampus and frontal cortex. CUS induced depression has been widely used for investigating the pathophysiology of depression and associated therapeutic interventions.24,25,26 Several studies suggest that CUS can induce long term behavioral disturbances that resemble the symptoms of clinical depression,22,26 and that CUS induced depression in animal models can be used for evaluating the efficacy of antidepressant candidates through behavioral tests including the sucrose preference, open field tests and shuttle-box test.24,25,27,28 The sucrose preference test is an indicator of anhedonia like behavioral change.25,26 Anhedonia, a core symptom of major depression among humans, is modeled by inducing a decrease in responsiveness to rewards reflected by reduced consumption of and/or preference for sweetened solutions,25.26 The results of the present study show that rats subjected to a 4 week period of CUS consumed less sucrose solution compared to non-stressed rats. Long term treatment with extract of BM significantly suppressed this behavioral change, which indicates the anti-depressant like action of the herbal product. In the open field test, normal animals usually show increased locomotor activity in a novel open field, which is driven by the instinct for exploration in an environment.22 However, after chronic stress, animals display decreased locomotor activity in a novel open field,29 which is indicative of a behavioral change that may reflect certain aspects of refractory depression, or loss of interest.22,24 In the shuttle-box testing, normal animals usually show decreased escape latency.3,4,15 However, after chronic stress treatment animals display increased escape latency and long term treatment with BM showed decreased escape latency as non-stressed control. In the present study, we found that exposure to CUS for a period of 4 weeks led to a significant reduction in the number of crossings and rearings in the open field test and escape deficits in shuttle-box test among the CUS exposed rats. However, the CUS induced decrease in sucrose consumption, locomotor activity and escape deficits were ameliorated by long term treatment with extract of BM during the course of CUS. Recent clinical studies of depression have paid special attention to the hippocampus and frontal cortex, which are brain regions structurally and functionally affected by stress responses and critically involved in the regulation of mood and learning/memory function.18,30 A causal relationship has been found between the incidence of major depressive disorders and neuronal atrophy in the hippocampus and frontal cortex.31,32 BDNF, which modulates neuronal plasticity, inhibits cell death cascades and increases the cell survival proteins that are responsible for the proliferation and maintenance of central nervous system neurons33; hence, it may be an important factor in the development and treatment of depression. In experimental studies, it has been found that a decrease in BDNF expression in the hippocampus and frontal cortex among animals exposed to chronic stress can be reversed by antidepressant treatment showed that conditional BDNF knockout mice displayed an increase in depression like behavior, which was assessed by the forced swim test and sucrose preference tests demonstrated that BDNF deficit can dampen the effects of antidepressants in mice exposed to chronic unpredictable mild stress. These studies provide evidence of the role of BDNF in depression, and indicate that the up regulation of BDNF expression may contribute to the action of antidepressants. Consistent with this view, the present study shows that CUS exposure decreases BDNF protein and mRNA levels in both the hippocampus and frontal cortex of depressed rats, while long term treatment by BM for the period of CUS exposure significantly reverses the CUS induced changes in behavior, BDNF protein and mRNA levels. The present results show that extract of BM (80-120 mg/kg) and imipramine both significantly increased BDNF expression in the hippocampus and frontal cortex of CUS treated rats.
In conclusion, our study demonstrates diminished activation of avoidance response which can be restored by treatment with BM extract of 80-120 mg/kg. The data presented herein suggest that selective increases in BDNF in specific brain regions, namely the hippocampus and frontal cortex, may be involved in the mechanism by which BM is effective as an anti-depressive therapeutic agent.
Acknowledgments
This work was financially supported by grants from and SERB [SR/SO/HS-57/2008] (DST) and LSRB/DRDO (Ministry of Defence, Govt. of India) [DLS/81/48222/LSRB-246/EPB/2012] Govt. of India.
References
1. Hankin BL. Adolescent depression: description, causes, and interventions. Epilepsy Behav 2006;8:102-114. PMID: 16356779.


2. Perahia DG, Quail D, Desaiah D, Montejo AL, Schatzberg AF. Switching to duloxetine in selective serotonin reuptake inhibitor non- and partialresponders: effects on painful physical symptoms of depression. J Psychiatr Res 2009;43:512-518. PMID: 18707693.


3. Banerjee R, Ghosh AK, Ghosh B, Mondal AC. Female-specific depression induction in learned-helplessness model of rats. Int J Bioeng Sci Tech 2011;2:51-59.
4. Banerjee R, Ghosh AK, Ghosh B, Mondal AC. Stress: the negative modulator of NGF. Res Rev: A J Life Sci 2011;1:1-9.
5. Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res 2005;53:129-139. PMID: 16024125.


6. Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol 2007;7:18-21. PMID: 17049922.


7. Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav 1997;56:131-137. PMID: 8981620.


8. Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brainderived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry 2005;57:1068-1072. PMID: 15860348.


9. Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, et al. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:1256-1260. PMID: 16647794.


10. Aydemir O, Deveci A, Taskin OE, Taneli F, Esen-Danaci A. Serum brain-derived neurotrophic factor level in dysthymia: A comparative study with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:1023-1026. PMID: 17433517.


11. Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 2003;54:70-75. PMID: 12842310.


12. Basterzi AD, Yazici K, Aslan E, Delialioglu N, Tasdelen B, Tot Acar S, et al. Effects of fluoxetine and venlafaxine on serum brain derived neurotrophic factor levels in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:281-285. PMID: 19110026.


13. Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig 2010;7:231-235.



14. Lee HY, Kim YK. Plasma brain-derived neurotrophic factor as a peripheral marker for the action mechanism of antidepressants. Neuropsychobiology 2008;57:194-199. PMID: 18679038.


15. Banerjee R, Ghosh AK, Ghosh B, Mondal AC. Chronic administration of Fluoxetine ameliorates depression: Enhanced BDNF and its receptor TrkB expressions with down stream signalling cascades ERK1/2 and Akt pathways. J Pharm Biomed Sci 2013;30:975-985.
16. Banerjee R, Ghosh AK, Ghosh B, Batabyal S, Mondal AC. Effects of chronic mild stress on brain derived neurotrophic and nerve growth factors in the rat hippocampus. Neurosci Res Lett 2012;3:29-34.
17. Zhang ZJ. Therapeutic effects of herbal extracts and constiturnts in animal models of psychiatric disorders. Life Sci 2004;75:1659-1699. PMID: 15268969.


18. Udupa KN, Singh RH. Clinical and Experimental Studies on Rasayana drugs and Panchkarma Therapy. New Delhi: Central Council for Research in Ayurveda and Siddha; 1995.
19. Hazra S, Banerjee R, Das BK, Ghosh AK, Banerjee TK, Hazra US, et al. Evaluation of antidepressant activity of Bacopa monnieri in rat: a study in animal model of depression. Drug Discov 2012;2:8-13.
20. Russo A, Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine 2005;12:305-317. PMID: 15898709.


21. Mao QQ, Ip SP, Ko KM, Tsai SH, Xian YF, Che CT. Effects of peony glycosides on mice exposed to chronic unpredictable stress: further evidence for antidepressant like activity. J Ethnopharmacol 2009;124:316-320. PMID: 19375493.


22. Luo DD, An SC, Zhang X. Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Res Bull 2008;77:8-12. PMID: 18579108.


23. Mao QQ, Ip SP, Tsai SH, Che CT. Antidepressant like effect of peony glycosides in mice. J Ethnopharmacol 2008;119:272-275. PMID: 18687393.


24. Katz RJ, Roth KA, Schmaltz K. Amphetamine and tranylcypromine in an animal model of depression: pharmacological specificity of the reversal effect. Neurosci Biobehav Rev 1981;5:259-264. PMID: 7196556.


25. Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10year review and evaluation. Psychopharmacology (Berl) 1997;134:319-329. PMID: 9452163.

26. Willner P. Chronic mild stress (CMS) revisited: consistency and behaviouralneurobiological concordance in the effects of CMS. Neuropsychobiology 2005;52:90-110. PMID: 16037678.


27. Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci 2007;80:1373-1381. PMID: 17289085.


28. Zhao Z, Wang W, Guo H, Zhou D. Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behav Brain Res 2008;194:108-113. PMID: 18655806.


29. D'Aquila PS, Peana AT, Carboni V, Serra G. Exploratory behaviour and grooming after repeated restraint and chronic mild stress: effect of desipramine. Eur J Pharmacol 2000;399:43-47. PMID: 10876021.


30. Qi X, Lin W, Li J, Li H, Wang W, Wang D, et al. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis 2008;31:278-285. PMID: 18586506.


31. Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull 2001;35:5-49. PMID: 12397885.
32. Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol 2004;14(Suppl 5):S481-S490. PMID: 15550346.


33. Yulug B, Ozan E, Gönül AS, Kilic E. Brain-derived neurotrophic factor, stress and depression: a minireview. Brain Res Bull 2009;78:267-269. PMID: 19111910.


Figure 1
Effect of BM treatment on the level of sucrose consumption in CUS exposed rats. values given are the mean±SEM (n=8). *p<0.05 as compared with the CUS group, †p<0.05 as compared with the Vehicle control group. CUS: chronic unpredictable stress, BM: graded doses of administered Bacopa monniera, IMI: imipramine hydrochloride, used as antidepressant drug, SEM: standard error of the mean.
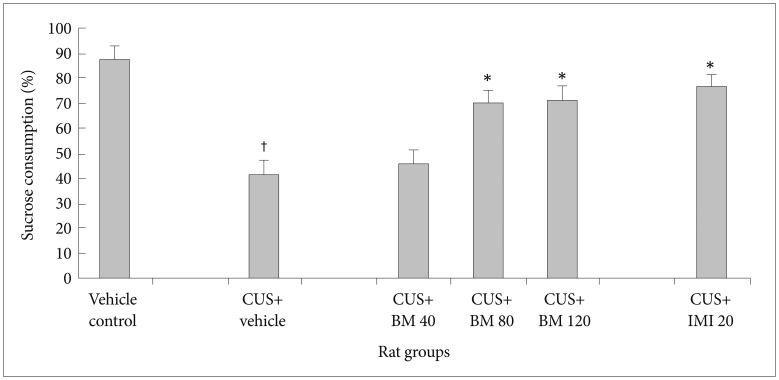
Figure 2
Effect of BM treatment on the number of crossings in the open field test in CUS exposed rats. Values given are the mean±SEM (n=8). *p<0.05 as compared with the CUS group, †p<0.05 as compared with the vehicle control group. CUS: chronic unpredictable stress, BM: graded doses of administered Bacopa monniera, IMI: imipramine hydrochloride, used as antidepressant drug, SEM: standard error of the mean.
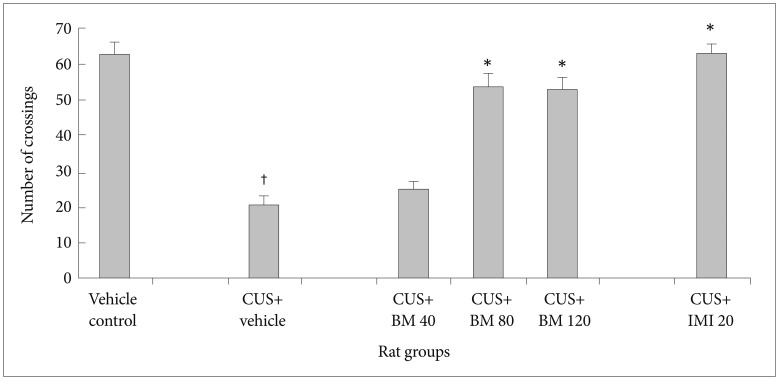
Figure 3
Effect of BM treatment on the number of rearings in the open field test in CUS exposed rats. Values given are the mean±SEM (n=8). *p<0.05 as compared with the CUS group, †p<0.05 as compared with the vehicle control group. CUS: chronic unpredictable stress, BM: graded doses of administered Bacopa monniera, IMI: imipramine hydrochloride, used as antidepressant drug, SEM: standard error of the mean.
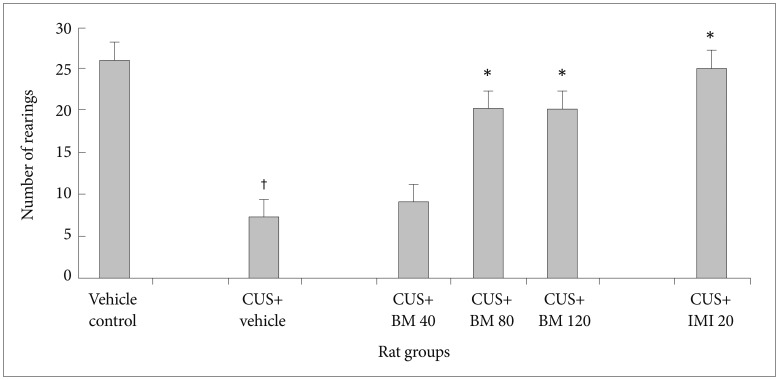
Figure 4
Effect of BM treatment on the escape latency in the Shuttle box escape test in CUS exposed rats. Values given are the mean±SEM (n=8). *p<0.05 as compared with the CUS group, †p<0.05 as compared with the vehicle control group. CUS: chronic unpredictable stress, BM: graded doses of administered Bacopa monniera, IMI: imipramine hydrochloride, used as antidepressant drug, SEM: standard error of the mean.
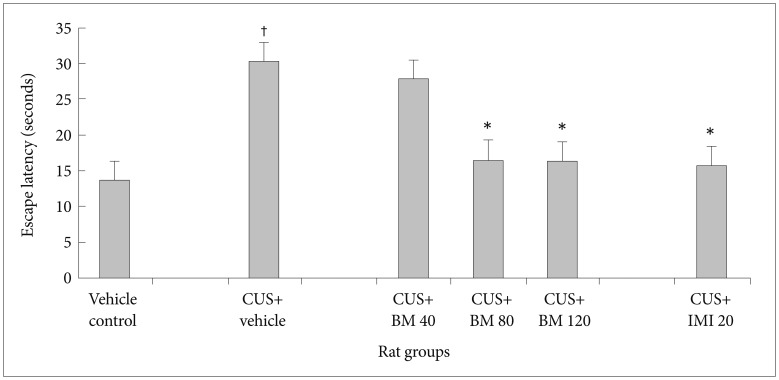
Figure 5
Effect of BM treatment on brain derived neurotrophin factor (BDNF) protein levels in the hippocampus and frontal cortex of CUS exposed rats. Values given are the mean±SEM (n=6). *p<0.001 as compared with the CUS group, †p<0.001 as compared with the Vehicle control group. CUS: chronic unpredictable stress, BM: graded doses of administered Bacopa monniera, IMI: imipramine hydrochloride, used as anti-depressant drug, SEM: standard error of the mean.
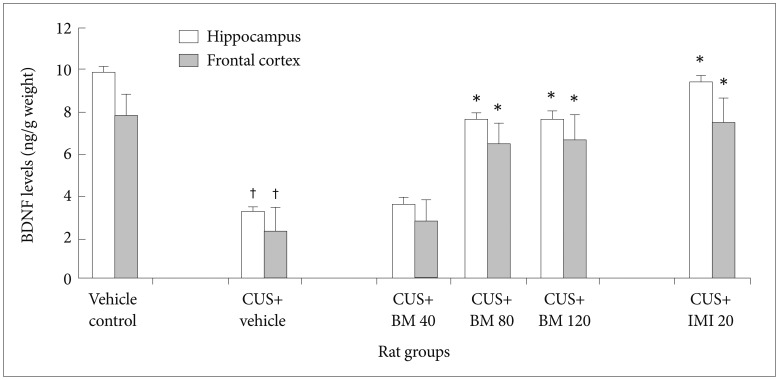
Figure 6
Effect of BM treatment on BDNF receptor TrkB protein levels in the hippocampus of CUS exposed rats. Values given are the mean±SEM (n=6). *p<0.001 as compared with the CUS group, †p<0.001 as compared with the Vehicle control group. CUS: chronic unpredictable stress, BM: graded doses of administered Bacopa monniera, IMI: imipramine hydrochloride, used as antidepressant drug, SEM: standard error of the mean.

Figure 7
Effect of BM treatment on BDNF receptor TrkB protein levels in the frontal cortex of CUS exposed rats. Values given are the mean±SEM (n=6). *p<0.001 as compared with the CUS group, †p<0.001 as compared with the Vehicle control group. CUS: chronic unpredictable stress, BM: graded doses of administered Bacopa monniera, IMI: imipramine hydrochloride, used as anti-depressant drug, SEM: standard error of the mean.
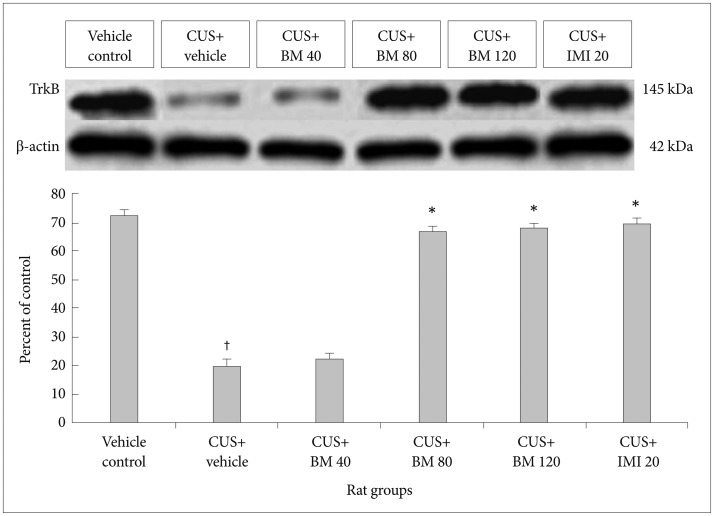
Figure 8
Effect of BM treatment on brain derived neurotrophin factor (BDNF) mRNA levels in the hippocampus and frontal cortex of CUS exposed rats. Values given are the mean±SEM (n=6). *p<0.001 as compared with the CUS group, †p<0.001 as compared with the Vehicle control group. CUS: chronic unpredictable stress, BM: graded doses of administered Bacopa monniera, IMI: imipramine hydrochloride, used as anti-depressant drug, SEM: standard error of the mean.