 |
 |
- Search
| Psychiatry Investig > Volume 19(5); 2022 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jung Hyun Lee, Eunsoo Moon. Data curation: Jeonghyun Park. Formal analysis: Min Yoon. Investigation: Jung Hyun Lee, Eunsoo Moon. Methodology: Eunsoo Moon, Min Yoon. Project administration: Jung Hyun Lee, Eunsoo Moon. Resources: Jung Hyun Lee, Eunsoo Moon, Jeonghyun Park, Chi Eun Oh, Yoo Rha Hong. Software: Jeonghyun Park. Supervision: Eunsoo Moon. Validation: Chi Eun Oh, Yoo Rha Hong. Visualization: Jung Hyun Lee, Eunsoo Moon. Writing—original draft: Jung Hyun Lee, Eunsoo Moon. Writing—review & editing: Jeonghyun Park, Chi Eun Oh, Yoo Rha Hong, Min Yoon.
Funding Statement
None
Figure 1.
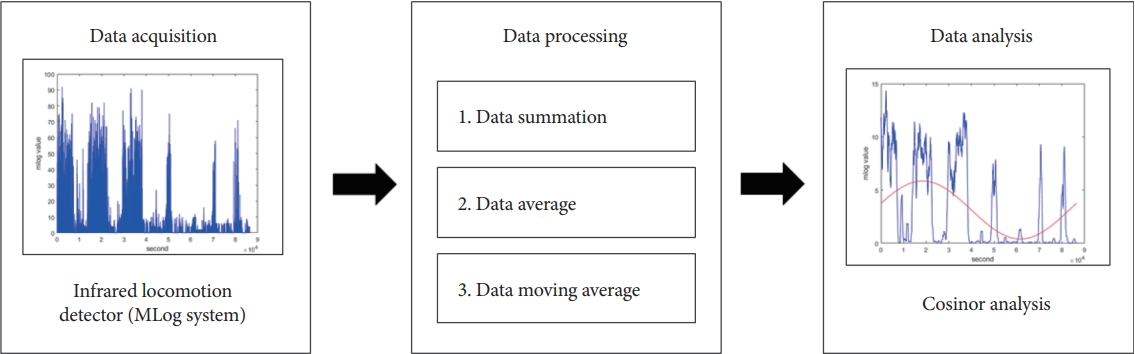
Figure 2.

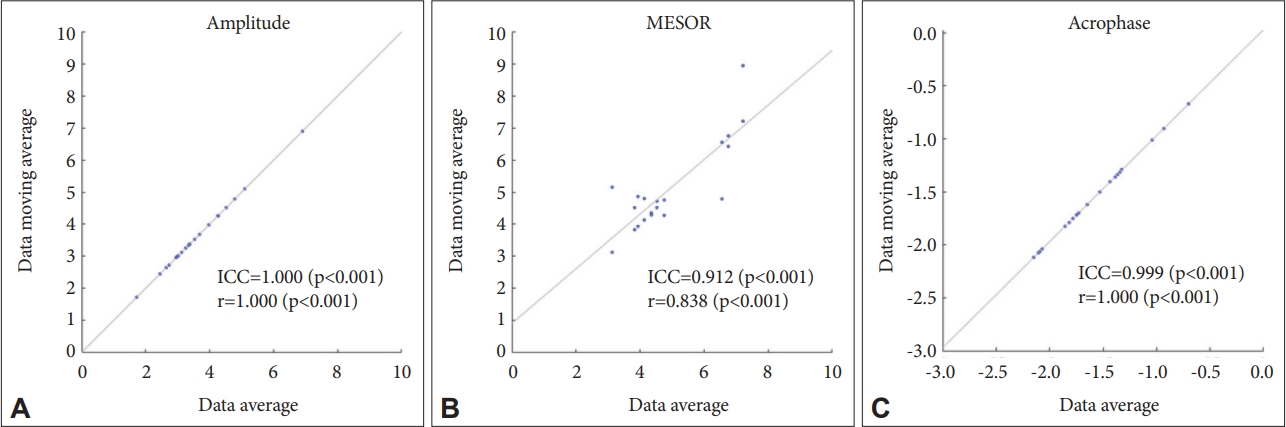
Figure 3.
REFERENCES







