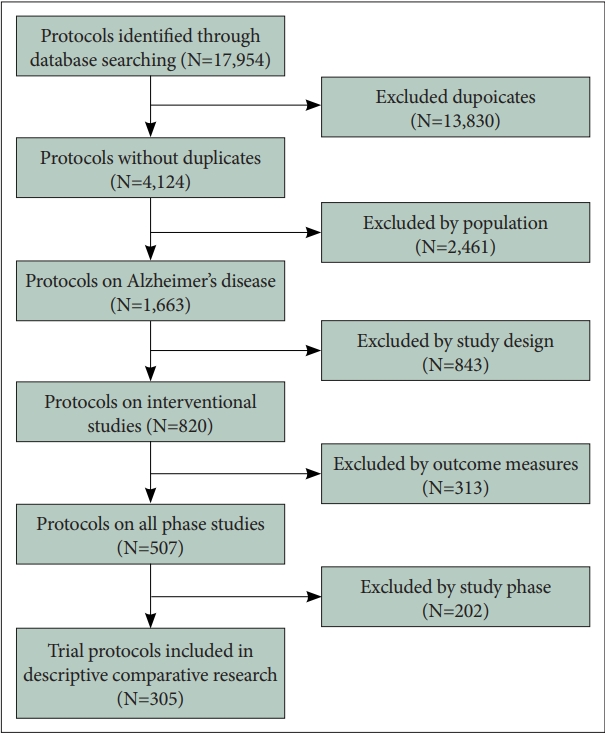
|
 |
- Search
| Psychiatry Investig > Volume 19(10); 2022 > Article |
|
Abstract
Objective
Methods
Results
Notes
Availability of Data and Material
The datasets generated or analyzed during the current study are available in the ClinicalTrials.gov repository, https://clinicaltrials.gov/.
Conflicts of Interest
Ki Woong Kim, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Ki Woong Kim, Riyoung Na, Jong Bin Bae. Data curation: Riyoung Na, Sue Hyun Jung. Formal analysis: Riyoung Na. Investigation: Riyoung Na, Sue Hyun Jung. Methodology: Ki Woong Kim, Riyoung Na. Project administration: Jong Bin Bae. Supervision: Ki Woong Kim. Writing—original draft: Riyoung Na, Jong Bin Bae. Writing—review & editing: Riyoung Na, Jong Bin Bae, Ki Woong Kim.
Funding Statement
None
Table 1.
|
CDISC TAUG-AD 2.0.1* |
Chi-square test |
|||
|---|---|---|---|---|
| Before (N=254) | After (N=51) | χ2 | p | |
| Intervention | 0.116 | 0.734 | ||
| Pharmacological | 231 | 45 | ||
| Nonpharmacological* | 23 | 6 | ||
| Study phase | 8.180 | 0.043 | ||
| Phase 1-2 | 14 | 6 | ||
| Phase 2 | 128 | 31 | ||
| Phase 2-3 | 12 | 5 | ||
| Phase 3 | 10 | 9 | ||
| Funder type | 2.303 | 0.316 | ||
| Public† | 54 | 13 | ||
| Industry | 179 | 31 | ||
| Public and industry | 21 | 7 | ||
Table 2.
|
CDISC TAUG-AD 2.0.1* |
p* | |||
|---|---|---|---|---|
| Before (N=254) | After (N=51) | |||
| Primary endpoint | 285 | 41 | ||
| Cognitive function | 177 (62.1) | 25 (61.0) | 0.889 | |
| Activity of daily living | 24 (8.4) | 0 | 0.053 | |
| Behavioral and psychological symptoms of dementia | 31 (10.9) | 9 (22.0) | 0.043 | |
| Global severity | 52 (18.2) | 7 (17.1) | 0.855 | |
| Others | 1 (0.4) | 0 | 0.704 | |
| Secondary endpoints | 705 | 149 | ||
| Cognitive function | 214 (30.4) | 64 (43.0) | 0.003 | |
| Activity of daily living | 141 (20.0) | 23 (15.4) | 0.199 | |
| Behavioral and psychological symptoms of dementia | 150 (21.3) | 24 (16.1) | 0.155 | |
| Global severity | 154 (21.8) | 34 (22.8) | 0.794 | |
| Others | 46 (6.5) | 4 (2.7) | 0.070 | |
| Not primary or secondary endpoints | 21 | 3 | ||
| Cognitive function | 7 (33.3) | 0 | 0.235 | |
| Activity of daily living | 4 (19.0) | 1 (33.3) | 0.569 | |
| Behavioral and psychological symptoms of dementia | 5 (23.8) | 0 | 0.342 | |
| Global severity | 2 (9.5) | 1 (33.3) | 0.243 | |
| Others | 3 (14.3) | 1 (33.3) | 0.408 | |
Table 3.
|
CDISC TAUG-AD 2.0.1* |
p* | |||
|---|---|---|---|---|
| Before (N=224) | After (N=41) | |||
| Listed in the CDISC TAUG-AD 2.0.1 | 398 | 89 | ||
| Alzheimer’s Disease Assessment Scale-Cognitive Subscale | 180 (45.2) | 28 (31.5) | 0.018 | |
| Mini-Mental State Examination | 117 (29.4) | 23 (25.8) | 0.503 | |
| Not listed in the CDISC TAUG-AD 2.0.1 | ||||
| Alzheimer’s Prevention Initiative Composite Cognitive Test | 1 (0.3) | 0 | 0.636 | |
| Cambridge Mental Disorders of the Elderly Examination | 1 (0.3) | 0 | 0.636 | |
| Cambridge Neuropsychological Test Automated Battery-Alzheimer’s Disease | 5 (1.3) | 1 (1.1) | 0.918 | |
| Clock Drawing Test | 2 (0.5) | 0 | 0.503 | |
| Cognitive Assessment Battery | 1 (0.3) | 0 | 0.636 | |
| Cognitive Drug Research Test Battery | 1 (0.3) | 0 | 0.636 | |
| Cognitive Failures Questionnaire | 1 (0.3) | 0 | 0.636 | |
| Computerized Test Battery for Cognition | 7 (1.8) | 1 (1.1) | 0.670 | |
| Confrontation Naming (Gremots) | 0 | 1 (1.1) | 0.034 | |
| Controlled Oral Word Association Test | 5 (1.3) | 1 (1.1) | 0.918 | |
| Corsi Block-Tapping Task | 0 | 1 (1.1) | 0.034 | |
| d2 Test of Attention | 0 | 1 (1.1) | 0.034 | |
| Delayed Word Recall Test (DWR) | 1 (0.3) | 0 | 0.636 | |
| Digit Cancellation Task (D-CAT) | 1 (0.3) | 0 | 0.636 | |
| Digit Span Task | 1 (0.3) | 1 (1.1) | 0.245 | |
| DMS 48 | 0 | 1 (1.1) | 0.034 | |
| Executive Abilities: Measures and Instruments for Neurobehavioral Evaluation and Research (EXAMINER) Battery | 1 (0.3) | 0 | 0.636 | |
| Free and Cued Selective Reminding Test (FCSRT) | 2 (0.5) | 0 | 0.503 | |
| Frontal Assessment Battery (FAB) | 3 (0.8) | 0 | 0.411 | |
| Grooved Pegboard Test (GPT) | 0 | 1 (1.1) | 0.034 | |
| Hopkins Verbal Learning Test (HVLT) | 3 (0.8) | 0 | 0.411 | |
| Isaacs Set Test (IST) | 1 (0.3) | 0 | 0.636 | |
| Maze Task | 1 (0.3) | 0 | 0.636 | |
| Mindstream Battery | 1 (0.3) | 0 | 0.636 | |
| Mini Mental Status (MMS) | 2 (0.5) | 0 | 0.503 | |
| Montreal Cognitive Assessment (MoCA) | 0 | 3 (3.4) | <0.001 | |
| Neuropsychological Test Battery (NTB) | 7 (1.8) | 3 (3.4) | 0.332 | |
| Poppelreuter-Ghent’s Overlapping Figures Test | 0 | 1 (1.1) | 0.034 | |
| Repeatable Battery for Assessment of Neuropsychological Status (RBANS) | 4 (1.0) | 1 (1.1) | 0.920 | |
| Rey Auditory Verbal Learning Test (RAVLT) | 0 | 1 (1.1) | 0.034 | |
| Rey-Osterrieth Complex Figure Test (ROCF) | 1 (0.3) | 1 (1.1) | 0.245 | |
| Savonix Digital Cognitive Assessment Platform | 0 | 1 (1.1) | 0.034 | |
| Severe Impairment Battery (SIB) | 13 (3.3) | 2 (2.2) | 0.615 | |
| Stroop Test | 1 (0.3) | 2 (2.2) | 0.030 | |
| Symbol Digit Modalities Test (SDMT) | 1 (0.3) | 1 (1.1) | 0.245 | |
| Test of Everyday Attention (TEA) | 0 | 1 (1.1) | 0.034 | |
| The Modified Mini-Mental State (3MS) | 2 (0.5) | 0 | 0.503 | |
| Trail Making Test (TMT) | 10 (2.5) | 4 (4.5) | 0.312 | |
| Verbal Fluency Test (VFT) | 9 (2.3) | 4 (4.5) | 0.237 | |
| Wechsler Adult Intelligence Scale (WAIS) | 8 (2.0) | 4 (4.5) | 0.172 | |
| Wechsler Memory Scale (WMS) | 3 (0.8) | 0 | 0.411 | |
| Zazzo’s Cancellation Test (ZCT) | 1 (0.3) | 0 | 0.636 | |
Table 4.
| Activities of daily living |
CDISC TAUG-AD 2.0.1* |
p* | |||
|---|---|---|---|---|---|
| Before (N=155) | After (N=23) | ||||
| Activities of daily living (function) | 169 | 24 | |||
| Listed in the CDISC TAUG-AD 2.0.1 | |||||
| Alzheimer’s Disease Cooperative Study-Activities of Daily Living-MCI | 6 (3.6) | 1 (4.2) | 0.880 | ||
| Disability Assessment for Dementia | 27 (16.0) | 1 (4.2) | 0.124 | ||
| Functional Activities Questionnaire | 2 (1.2) | 4 (16.7) | <0.001 | ||
| Not listed in the CDISC TAUG-AD 2.0.1 | |||||
| Alzheimer’s Disease Cooperative Study-Activities of Daily Living | 98 (58.0) | 10 (41.7) | 0.132 | ||
| Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory for Severe AD | 6 (3.6) | 1 (4.2) | 0.880 | ||
| Alzheimer’s Disease Cooperative Study-Instrumental Activities of Daily Living | 3 (1.8) | 3 (12.5) | 0.005 | ||
| Alzheimer’s Disease Functional Assessment and Change Scale | 2 (1.2) | 0 | 0.592 | ||
| Bristol Activities of Daily Living Scale | 1 (0.6) | 0 | 0.701 | ||
| Dependence Scale | 4 (2.4) | 0 | 0.446 | ||
| Direct Assessment of Functional Status | 1 (0.6) | 0 | 0.701 | ||
| Interview for Deterioration in Daily Living Activities in Dementia | 1 (0.6) | 0 | 0.701 | ||
| Katz Index | 2 (1.2) | 0 | 0.592 | ||
| Korean Instrumental Activities of Daily Living | 2 (1.2) | 2 (8.3) | 0.021 | ||
| Lawton Instrumental Activities of Daily Living | 9 (5.3) | 1 (4.2) | 0.810 | ||
| Minimum Data Set-Activities of Daily Living | 1 (0.6) | 0 | 0.701 | ||
| Partner-Patient Questionnaire for Shared Activities | 2 (1.2) | 0 | 0.592 | ||
| Physical Self-Maintenance Scale | 1 (0.6) | 0 | 0.701 | ||
| Seoul-Instrumental Activities of Daily Living | 1 (0.6) | 1 (4.2) | 0.106 | ||
Table 5.
| Global severity |
CDISC TAUG-AD 2.0.1* |
p* | |||
|---|---|---|---|---|---|
| Before (N=176) | After (N=30) | ||||
| Global severity | 208 | 42 | |||
| Listed in the CDISC TAUG-AD 2.0.1 | |||||
| Clinical Dementia Rating | 66 (31.7) | 17 (40.5) | 0.272 | ||
| Clinical Global Impressions | 39 (18.8) | 4 (9.5) | 0.003 | ||
| Not listed in the CDISC TAUG-AD 2.0.1 | |||||
| Alzheimer’s Disease Assessment Scale | 2 (1.0) | 0 | 0.523 | ||
| Alzheimer’s Disease Composite Score | 1 (0.5) | 1 (2.4) | 0.207 | ||
| Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change | 40 (19.2) | 6 (14.3) | 0.451 | ||
| Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change for MCI score | 0 | 1 (2.4) | 0.026 | ||
| Clinician’s Interview-Based Impression of Change-Plus Caregiver | 49 (23.6) | 7 (16.7) | 0.329 | ||
| Global Deterioration Scale | 3 (1.4) | 2 (4.8) | 0.161 | ||
| Integrated Alzheimer’s Disease Rating Scale | 2 (1.0) | 3 (7.1) | 0.009 | ||
| Nurses’ Observation Scale for Geriatric Patients | 1 (0.5) | 0 | 0.653 | ||
| Participant’s Global Impression of Change | 5 (2.4) | 1 (2.4) | 0.993 | ||
Table 6.
| Behavioral and psychological symptoms of dementia |
CDISC TAUG-AD 2.0.1* |
p* | |||
|---|---|---|---|---|---|
| Before (N=142) | After (N=28) | ||||
| Behavioral and psychological symptoms of dementia | 186 | 33 | |||
| Listed in the CDISC TAUG-AD 2.0.1 | |||||
| Geriatric Depression Scale | 4 (2.2) | 4 (12.1) | 0.005 | ||
| Neuropsychiatric Inventory | 116 (62.4) | 20 (60.6) | 0.848 | ||
| Not listed in the CDISC TAUG-AD 2.0.1 | |||||
| Alzheimer’s Disease Assessment Scale-Noncognitive Subscale | 2 (1.1) | 0 | 0.550 | ||
| Apathy Evaluation Scale | 2 (1.1) | 0 | 0.550 | ||
| Apathy Inventory | 3 (1.6) | 0 | 0.463 | ||
| Beck Depression Inventory-II | 1 (0.5) | 0 | 0.673 | ||
| Behavioral Pathology in Alzheimer’s Disease | 10 (5.4) | 0 | 0.173 | ||
| Brief Psychiatric Rating Scale | 4 (2.2) | 0 | 0.395 | ||
| Cohen-Mansfield Agitation Inventory | 14 (7.5) | 6 (18.2) | 0.050 | ||
| Cornell Scale for Depression in Dementia | 17 (9.1) | 0 | 0.071 | ||
| Frontal Systems Behavior Scale | 2 (1.1) | 0 | 0.550 | ||
| Hamilton Depression Scale | 3 (1.6) | 1 (3.0) | 0.573 | ||
| Modified Crichton Royal Behavioural Rating Scale | 2 (1.1) | 0 | 0.550 | ||
| Montgomery-Asberg Depression Rating Scale | 2 (1.1) | 1 (3.0) | 0.373 | ||
| Neurobehavioral Rating Scale | 1 (0.5) | 0 | 0.673 | ||
| Pittsburgh Agitation Scale | 0 | 1 (3.0) | 0.017 | ||
| Positive and Negative Syndrome Scale | 1 (0.5) | 0 | 0.673 | ||
| Starkstein Apathy Scale | 2 (1.1) | 0 | 0.550 | ||
Table 7.
| Other symptoms |
CDISC TAUG-AD 2.0.1* |
p* | |||
|---|---|---|---|---|---|
| Before (N=40) | After (N=4) | ||||
| Others | 50 | 5 | |||
| Listed in the CDISC TAUG-AD 2.0.1 | |||||
| Modified Hachinski Ischemic Scale | 1 (2.0) | 0 | 0.750 | ||
| Not listed in the CDISC TAUG-AD 2.0.1 | |||||
| Alzheimer’s Disease-Related Quality of Life | 1 (2.0) | 0 | 0.750 | ||
| Dementia Quality of Life | 6 (12.0) | 1 (20.0) | 0.609 | ||
| European Quality of Life-5 Dimensions | 22 (44.0) | 1 (20.0) | 0.230 | ||
| Hachinski Ischemia Scale (HIS) | 0 | 1 (20.0 | 0.001 | ||
| Quality of Life in Alzheimer’s Disease (QoL-AD) | 17 (34.0) | 1 (20.0) | 0.525 | ||
| The Short Form 36 Health Survey Questionnaire (SF-36) | 0 | 1 (20.0) | 0.001 | ||
| The World Health Organization Quality of Life-BREF (WHOQOL-BREF) | 1 (2.0) | 0 | 0.750 | ||
| Timed Up and Go (TUB) | 2 (4.0) | 0 | 0.133 | ||
REFERENCES
-
METRICS

-
- 0 Crossref
- Scopus
- 2,563 View
- 53 Download
-
Incidence and Course of Depression in Patients with Alzheimer's Disease2017 May;14(3)