Association of a History of Sleep Disorder With Risk of Mild Cognitive Impairment and Alzheimer’s Disease Dementia
Article information
Abstract
Objective
We explored whether a history of sleep disorder affected a current diagnosis of cognitive impairment and clinical conversion in a non-demented elderly population.
Methods
Comprehensive clinical data collected as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) was analyzed. A history of sleep disorder was recorded in the recent ADNI medical database. Standard clinical and neuropsychological tests were performed both at baseline and follow-up visit. Multiple logistic regression analysis was performed after adjusting for age, sex, education, apolipoprotein E ε4 status, vascular risk score, body mass index, Geriatric Depression Scale score, and use of sleeping pills.
Results
A total of 391 cognitively normal individuals, 303 with early mild cognitive impairment (MCI) and 364 with late MCI were included. Sleep disorder history was significantly associated with an increased risk of MCI but not with clinical conversion. A history of insomnia or obstructive sleep apnea (OSA) significantly increased the risk of MCI, but only an OSA history predicted progression to Alzheimer’s disease (AD) dementia.
Conclusion
Our findings suggest that a sleep disorder history usefully aids early detection of cognitive impairment and emphasize that such sleep disorder, particularly OSA, is important as potential target for AD prevention.
INTRODUCTION
Disturbed sleep is closely associated with Alzheimer’s disease (AD) dementia [1-3]. AD patients exhibit more sleep fragmentation and less slow-wave sleep than do normal elderly people [4]. Conversely, self-reported sleep problems [5] and sleep fragmentation measured by actigraphy [2] are associated with an increased risk of incident AD. A recent study highlighted the role played by sleep disturbance as a risk factor for AD; poor sleep quality during midlife increased cerebral beta-amyloid deposition in cognitively normal (CN) older adults [6].
Of the many observational studies investigating the effect of sleep on cognitive impairment, those enrolling patients with specific sleep disorders (e.g., insomnia, obstructive sleep apnea [OSA], and restless legs syndrome [RLS]) have been few in number compared to works on sleep problem such as poor sleep quality and short sleep duration [7]. Most studies exploring the effects of sleep disorders on cognition included OSA subjects [7]. Some studies reported that elderly people with sleep-disordered breathing were at risk of cognitive impairment [8,9], but others found no such association [10,11]. Recent meta-analyses suggested that sleep apnea is associated with increased risks of cognitive impairment [12] and AD [13]. However, the mechanisms in play remain unclear [14].
Most sleep disorders feature a disrupted sleep architecture, but they also exhibit unique features, such as intermittent cerebral hypoxia and intrathoracic pressure changes in OSA [8,15] and brain iron insufficiency and altered dopaminergic function in RLS [16]. Therefore, an exploration of the relationships between cognitive status and sleep disorders, in addition to disturbed sleep, may elucidate novel causative mechanisms.
Here, we evaluated whether a history of sleep disorder affected the risk of mild cognitive impairment (MCI) and clinical conversion to AD dementia in a non-demented elderly population. We analyzed the comprehensive clinical data collected as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and this allowed us to control for confounding factors such as depression and the use of sleeping pills.
METHODS
Study design and participants
All data were those of the ADNI database (adni.loni.usc. edu). The ADNI was launched in 2003 as a public-private partnership led by principal investigator Michael W. Weiner, MD. The primary goal was to explore whether serial magnetic resonance imaging, positron emission tomography, measurements of biological markers, and clinical and neuropsychological assessments, could be combined to measure the progression of MCI and early AD. The study protocol was approved by the Institutional Review Boards of all participating institutions and written informed consent was obtained from all participants. For up-to-date information, see www.adni-info.org.
We included CN, early MCI (EMCI), and late MCI (LMCI) participants for whom complete data, collected during the ADNI 1, ADNI GO, and ADNI 2 studies, were available. Data from the ADNI 3 study were not used because the format used to evaluate medical history changed from that employed earlier [17]. CN subjects evidenced no memory problem apart from those common in normal aged subjects, normal memory function as revealed by the Wechsler Logical Memory II subscale, and normal cognition based on the absence of significant impairments in cognitive function or the activities of daily living. The Mini-Mental State Examination (MMSE) scores were 24–30 and the Clinical Dementia Rating (CDR) scores 0. All EMCI and LMCI individuals met the current consensus criteria for amnestic MCI [18]: a memory complaint made by the subject or a partner, objective memory loss as measured by the education-adjusted scores on the Wechsler Memory Scale Logical Memory II subscale, absence of significant impairment in other cognitive domains, essentially preserved activities of daily living, and no dementia. The memory scores of EMCI subjects lay approximately 0.5 to 1.5 standard deviations below the mean CN score, and the memory performance of LMCI individuals was below that of EMCI subjects [19]. EMCI and LMCI individuals had MMSE scores of 24–30 and CDR scores of 0.5. Subjects were considered to have progressed to AD dementia if they met the National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD (MMSE scores of 20–26 and a CDR of 0.5 or 1.0) [20] at any follow-up visit. Subjects with any significant neurologic disease other than AD were excluded. The detailed inclusion/exclusion criteria are at www.adni-info.org.
Assessment
All participants underwent a standardized clinical evaluation based on the study protocol. The status of all of six vascular risk factors (hypertension, diabetes, dyslipidemia, transient ischemic attack, stroke, and coronary artery disease) was systematically evaluated and the vascular risk score (VRS) calculated as the total number of such factors [21]. Depressive symptoms were assessed using the Geriatric Depression Scale (GDS) [22]. The MMSE score, body mass index (BMI), and apolipoprotein E (APOE) genotype were recorded. APOE ε4 (APOE4) carrier status was considered positive if least one ε4 allele was present.
History of sleep disorder
Sleep disorder history was evaluated using recent entries into the ADNI medical history database. At the baseline visits, patients or caregivers reported clinically significant histories of medical problems. We selected the three most common sleep disorders (insomnia, OSA, and RLS) in the US population23 and searched for histories of sleep disorder using the keywords: “insomnia,” “sleep,” “(sleep) disturbance,” and “(sleep) difficulty” for insomnia; “(sleep) apnea,” “OSA,” and “CPAP” for OSA; and “RLS” and “restless legs” for RLS. We double-checked all records made in the “psychiatric,” “neurological,” “respiratory,” and “other” system domains for data reflecting sleep disorders. Sleeping pill use was defined as the taking of benzodiazepines or Z-drugs at the baseline visits. The keywords were: eszopiclone (Lunesta), zopiclone (Imovane), zolpidem (Ambien), zaleplon (Sonata), alprazolam (Xanax), diazepam (Valium), lorazepam (Ativan), temazepam (Restoril), clonazepam (Klonopin), triazolam (Halcion), flunitrazepam (Rohypnol), oxazepam (Serax), estazolam (Prosom), flurazepam (Dalmane), and quazepam (Doral).
Statistical analysis
Comparison of continuous variables between cognitive diagnoses was done via an analysis of variance (ANOVA) with the post-hoc Tukey test. The chi-squared test was used to compare categorical variables. Multiple logistic regression analyses were performed to explore associations between a history of sleep disorder and the current cognitive diagnosis (CN vs. MCI) after controlling for age, sex, education, APOE4 status, VRS, BMI, GDS, and use of sleeping pills. We used multiple logistic regression analyses to evaluate the association between a history of a sleep disorder and clinical conversion to a moreimpaired cognitive diagnosis (i.e., CN to MCI or AD dementia; EMCI or LMCI to AD dementia). The whole-group analyses added the cognitive diagnoses (CN, EMCI, and LMCI) to the covariates used in previous analyses, and subgroup analyses combined the baseline MMSE score to adjust for severity of cognitive impairment. Clinical conversion survival curves were drawn using the Kaplan–Meier method. A Cox’s proportional hazards model featuring stepwise forward selection was used to evaluate clinical conversion by historical OSA status. All statistical analyses were performed using IBM SPSS Statistics ver. 21 (IBM Co., Armonk, NY, USA), and the level of statistical significance was set as two-tailed p<0.05.
RESULTS
Demographic and clinical characteristics
Participant characteristics are listed in Table 1. A total of 1,058 subjects (391 CN, 303 EMCI, 364 LMCI) were included in this study. The mean age was 73.03 years and the proportion of males was 55.5%. We found significant amonggroup differences in terms of age, sex, APOE4 carrier status, BMI, MMSE and GDS scores, clinical conversion, a history of sleep disorder (especially OSA), and sleeping pill use. Overall, 48 (12.3%) CN subjects, 74 (24.4%) EMCI individuals, and 56 (15.4%) LMCI participants had histories of sleep disorders (usually OSA). The mean follow-up period was 4.2 years, ranging from 0.4 to 15.0 years. Further, 86 (22.0%) subjects that were of CN status at baseline progressed to MCI (EMCI or LMCI), and 52 (17.2%) with EMCI and 188 (51.6%) with LMCI to AD dementia.
Association between a history of sleep disorder and a current cognitive diagnosis (cognitively normal vs. mild cognitive impairment)
Multiple logistic regression analyses revealed that a history of a sleep disorder was significantly associated with an increased risk of MCI after controlling for age, sex, educational level, APOE4 status, VRS, BMI, GDS, and sleeping pill use as shown in Table 2 (odds ratio [OR]=1.956, 95% confidence interval [CI]=1.312–2.917, p<0.001). On further analyses, histories of insomnia (OR=1.910, 95% CI=1.061–3.436, p=0.031) and OSA (OR=2.172, 95% CI=1.285–3.669, p=0.004) were significantly associated with an increased risk of MCI, but an RLS history was not.
Association between a history of sleep disorder and clinical conversion
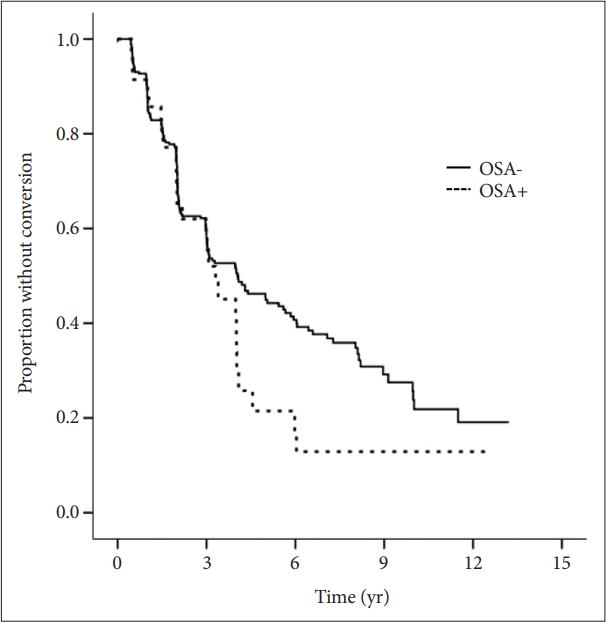
Table 3 shows the results of a multiple logistic regression analysis in terms of clinical conversion to a more advanced diagnosis as the dependent variable in whole cognitive group. Only an OSA history was significantly associated with a high risk of clinical conversion after adjustment for age, sex, and the cognitive diagnosis (OR=1.772, 95% CI=1.113–2.820, p=0.016), and the association remained significant after additionally controlling for educational level, APOE4 status, VRS, BMI, GDS, and use of sleeping pills (OR=2.073, 95% CI=1.282– 3.354, p=0.003). Subgroup analyses revealed that a history of OSA was significantly associated with an increased risk of clinical conversion in the LMCI group (OR=4.308, 95% CI=1.845–10.055, p=0.001) and the MCI (combination of EMCI and LMCI subjects) group (OR=1.830, 95% CI=1.087–3.082, p=0.023) after controlling for the same covariates (except the cognitive diagnosis replaced by the MMSE) (Table 4). A sleep history was not associated with the risk of clinical conversion in the CN and EMCI groups. We used a Cox’s proportional hazards model controlling for the same covariates to evaluate the associations between historical sleep disorder status and clinical conversion, and drew Kaplan-Meier survival curves (Figure 1). An OSA history significantly increased the risk of AD dementia conversion in LMCI subjects (OR=1.623, 95% CI=1.061–2.484, p=0.026) but not in any other cognitive group.

Multiple logistic regression analysis with clinical conversion as dependent variable in whole cognitive group

Multiple logistic regression analysis with clinical conversion as dependent variable in cognitive subgroup
DISCUSSION
A sleep disorder history may usefully predict MCI but not clinical conversion to AD dementia. In analyses employing specific sleep disorders, both insomnia and OSA were associated with increased risks of MCI, but only prior OSA predicted clinical conversion to AD dementia. To the best of our knowledge, this is the first study to explore the effects of overall past sleep disorder status on a current cognitive diagnosis or clinical conversion, although there have been studies targeting specific sleep disorders.
Our principal finding is that prior OSA increases the risk of AD dementia conversion in MCI individuals. These results are supported by those of several studies that used the dementia and MCI risks as outcome variables. Older females exhibiting sleep-disordered breathing were more likely to develop MCI or dementia [8], and older males with sleep apnea were at an increased risk of AD [24]. A population-based study also found that patients with sleep apnea were at greater risk of dementia [25]. Although the linkage between OSA and cognitive decline remains unclear, increasing evidence suggests that hypoxia rather than sleep fragmentation might play key role to increase the risk of cognitive impairment [8,9]. Chronic intermittent hypoxemia triggers vasculopathy, blood–brain barrier disruption, and changes in synaptic plasticity that lead cognitive decline [26,27]. Our findings also support the critical role played by hypoxia in terms of cognitive decline by showing that an OSA history predicted AD conversion while insomnia, which reflects sleep disruption rather than hypoxia, did not.
We also found that an insomnia history was associated with an increased risk of MCI. Assuming the presence of MCI at baseline indicates conversion from CN to MCI prior to baseline visit, our findings provide evidence that subjects with an insomnia history were more likely to develop MCI than were those lacking such histories. Although no significant association was found between insomnia history and MCI conversion in CN subjects during the follow-up period, this may be due to the relatively short period of time. In subjects with MCI, an insomnia history was not associated with a risk of clinical conversion to AD dementia during follow-up, suggesting that insomnia was a weak trigger of AD dementia progression. Contrary to our findings, several prospective cohort studies that measured poor sleep through actigraphy [2,28] or questionnaires [29,30] reported that disturbed sleep increased the AD risk. This may reflect the fact that the severity of sleep disturbance varies by the measurement method employed. Benzodiazepines and benzodiazepine-receptor agonists are commonly used to treat insomnia [31], and these medications reduce sleep latency and awakenings, and increase total sleep time and sleep quality [32]. As medicated subjects are more likely to remember and report insomnia histories, it may be that the real sleep disturbances were milder in subjects reporting insomnia histories than in those for whom sleep fragmentation was directly measured. We sought to control for the effects of sleeping pills, but only baseline data (thus not data at the times of insomnia) were available. Lastly, it should also be considered that recall bias and under-reporting of insomnia may weaken the association between such a history and clinical conversion.
Evidence that a history of RLS increase the risk of MCI or AD dementia was not found. An RLS may increase sleep latency and fragmentation [33], but the published data are contradictory. RLS subjects evidenced cognitive deficits [34,35] but were at no significant risk of cognitive disorder [36]. However, this may reflect under-reporting of data on RLS cases, as an RLS is often confused with periodic limb movement disorder [33].
Our study was conducted in a well-defined longitudinal cohort. We tried to minimize the possibility of reverse causality by treating any history of a sleep disorder, that occurred long before the time of study data collection, as an independent variable and then tracking diagnostic progression over an adequate time. The comprehensive clinical data collection allowed us to control for various sleep-related confounders, particularly depression and the use of sleeping pills. However, our study had several limitations. First, a history of sleep disorder was self-reported based on individual recall in the ADNI cohort. In particular, self-reported insomnia may include heterogeneous conditions such as insomnia disorder, insomnia symptom and insomnia secondary to other causes. To minimize recall error, we included only a non-demented population. If recall bias is random, it is likely that the relationships between a sleep disorder history and a current cognitive diagnosis were in fact underestimated. Therefore, our findings are unlikely to be attributable to chance, although caution is required when interpreting non-significant results. Second, the use of continuous positive airway pressure (CPAP), a standard OSA therapy [37], was not reliably recorded. Although CPAP use was indeed noted, this did not truly reflect OSA treatment because compliance data were lacking and the reports covered historical events rather than real-time experiences. Our findings thus suggest that an OSA history increased the risk of AD dementia progression regardless of OSA treatment status.
In conclusion, our findings suggest that a sleep disorder history usefully aids the early detection of cognitive impairment and emphasize that sleep disorders (particularly OSA) should be targeted to prevent AD. Utilizing a history of sleep disorder has the advantage of being feasible and easy to use in clinical practice. Additional longitudinal studies on the utility of objective sleep measurements are still needed.
Notes
Availability of Data and Material
Data used in this study are available at the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu).
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: all authors. Data curation: Young Min Choe. Formal analysis: Young Min Choe, Jee Wook Kim. Investigation: Young Min Choe, Guk-Hee Suh. Methodology: Young Min Choe. Software: Young Min Choe. Supervision: Jee Wook Kim. Validation: Guk-Hee Suh, Jee Wook Kim. Writing—original draf: Young Min Choe. Writing—review & editing: Guk-Hee Suh, Jee Wook Kim.
Funding Statement
This study was supported by Hallym University Research Fund (HURF2020-34). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.