|
 |
- Search
| Psychiatry Investig > Volume 19(12); 2022 > Article |
|
Abstract
Objective
The current study aims to find out the potential reasons why most schizophrenia patients have a relatively low sensitivity to the classification of emotional facial expressions.
Methods
By using an emotional categorical perception task, eighty-three schizophrenia patients and seventy-one healthy adults are provided with morphed emotional continuums with two emotional facial expressions (a positive emotional valence: happy; a negative emotional valence: sad).
Results
Through comparing the difference between schizophrenia patients and healthy adults in the processes of estimating facial expressions with ambiguous emotions, we find that the pattern of emotional categorical perception for schizophrenia patients is significantly different from that of healthy controls when they process signals on the local facial areas. Compared to healthy people, schizophrenia patients have a significantly separate classification pattern in processing emotional signals between the eyes and mouth regions. It indicates that compared to healthy adults, schizophrenia patients have larger conflicts in integrating emotional signals from different facial areas. To overcome conflicts, more cognitive resources are required. Unfortunately, the lack of cognitive resources leads to the failure of integration, which further increases the difficulty of estimating facial expressions with ambiguous emotions, and finally leads to the relatively low sensitivity of emotional facial expressions classification.
Estimation of emotional facial expressions is an essential ability in daily life. However, this key ability has been damaged for the population of schizophrenia patients. Numerous previous studies have proved that abnormal perceptions of emotional facial expressions as well as the relatively low sensitivity to the classification of emotional facial expressions are core features of schizophrenia patients [1-6]. Schizophrenia patients are unable to quickly and accurately estimate emotions presented by ambiguous facial expressions [7]. Namely, when the emotional intensity of some expressions is weak, schizophrenia patients are unable to estimate these expressions as quickly and accurately as healthy people. The deficit often obstructs schizophrenia patients’ empathy and social communications [8-12]. Currently, there are two views to explain the mechanism of the deficit.
Some researchers suggested that the deficit results from a cognitive disability of emotions, namely an emotion-specific deficit [13]. They proposed that schizophrenia patients have specific deficits in encoding or decoding other’s emotions [14]. Others advocated that the deficit is a result of an integrated deficit of signals on facial areas. It belongs to a general cognitive deficit involving the effective integration of emotion and cognition [15]. This controversy has been going on for many years.
Recently, according to the finding that “the local features and structural information are the main factors affecting social perceptions of faces,” some studies indirectly proposed a new thought to deal with this controversy [16-18]. Specifically, the new thought is to investigate emotional classification patterns of schizophrenia patients in different facial areas. To illustrate the categorical perception of emotional facial expressions in healthy people, Wegrzyn et al. [18] instructed participants to classify emotional facial expressions with an emotional categorical perception task, and found that healthy adults can estimate facial expressions with ambiguous emotions in a relatively stable way, no matter what kind of facial area is processed. In other words, healthy adults always classify a facial expression with ambiguous emotions as having the same emotion, despite observing the eye region, mouth region, or the whole face respectively. It indicates that healthy adults’ categorical perception of emotional facial expressions is relatively stable regardless of processing whatever signals on the facial area. At bottom, their abilities to integrate the signals of different facial areas are intact. However, it is still unclear whether schizophrenia patients also have a relatively stable categorical perception of emotional facial expressions when they process signals on different facial areas. Through investigating this, the abovementioned controversy of whether the relatively low sensitivity to the classification of emotional facial expressions in schizophrenia patients is a cognitive disability of emotions or an integrated deficit of signals on different facial areas can be deconstructed to some extent.
Categorical perception of emotional facial expressions refers to a phenomenon of perception that individuals tend to make perceptual categorizations among emotional facial expressions when those facial expressions belong to different emotional categories rather than the same emotional category [19,20]. Normally, the emotional categorical perception task is utilized to investigate individuals’ categorical perception of emotional facial expressions. In this task, stimuli are gradually varied continuums of facial expressions. A continuum is created by two emotional facial expressions pictures. Each picture includes a facial expression image with a certain emotional valence and a 100% emotional intensity. The two pictures, called prototype pictures, are at the two endpoints of the continuum. Between the two endpoints, other pictures of emotional facial expressions are generated by morphing the two prototype pictures through computer software. Specifically, the emotional intensity of these morphed pictures of emotional facial expressions gradually varies between 0% and 100% compared to the emotion contained in the picture of left endpoints of the continuum. The emotional intensity increment is equal between every picture, it is often 10%. Consequently, the continuum gradually varies from one emotional facial expression to the other. In the task, observers’ classification boundary (also called “shift point”) and the sensitivity to the classification of emotional facial expressions can be calculated by a logistic function. The “shift point” indicates the position on which facial expressions with a certain emotion start to change to the other one in observers’ subjective views. It is also the point on the continuum at which the most likely choice of emotion shifts from an emotion to the other one. The sensitivity to the classification of emotional facial expressions refers to observers’ ability to classify the two emotional facial expressions. The general logistic function can be represented by the following simplest equation: Y=1/(1+e-x). According to different data types, the general logistic function has many variants.
Through providing schizophrenia patients with signals on different facial areas and instructing them to conduct emotional categorical perception tasks, we can clarify the difference of the classification between various conditions of facial areas. Namely, we can acquire schizophrenia patients’ pattern of emotional facial expressions classification under the condition of processing different facial signals. Subsequently, healthy adults’ classification pattern can also be acquired by this task, and the difference of classification patterns between schizophrenia patients and healthy adults can be analyzed. If schizophrenia patients and healthy adults have a similar emotional classification pattern in different facial areas, the relatively low sensitivity to the classification of emotional facial expressions is due to the cognitive disability of emotions. In contrast, if schizophrenia patients and healthy adults have a similar emotional classification pattern under the condition of observing a whole face, but different emotional classification pattern under the condition of local facial areas, then we can conclude that the relatively low sensitivity to the classification of emotional facial expressions arises from patients’ integrated deficit of signals on facial areas.
To date, few studies have investigated the abovementioned debate from the perspective of emotional categorical perceptions. In the current study, we used an emotional categorical perception task with different facial areas. Specifically, we used three “positive emotional valence-negative emotional valence” emotional continuums (“happy-sad” continuums). They are the continuum with upper half of facial area (mainly eye region), the continuum with lower half of facial area (mainly mouth region), and the continuum with whole faces. The aim of the current study is to deconstruct the mechanism of abnormal categorical perception of emotional facial expressions in schizophrenia patients by adopting this approach.
We assume that emotional categorical perception of schizophrenia patients will show some pathological characteristics during the processing of estimating emotional facial expressions. Specifically, there are two possible results. First, if the standpoint of cognitive disabilities of emotions is right, the similar classification boundary will exist between schizophrenia patients and healthy adults regardless what facial areas is observed. Oppositely, if the viewpoint of integrated deficit of signals on facial areas is right, significantly different classification boundary will occur between schizophrenia patients and healthy adults. Specifically, compared with healthy adults, schizophrenia patients would perceive more negative emotions when they observe ambiguous facial expressions in eye regions. In contrast, they would perceive more positive emotions when they observe ambiguous facial expressions in mouth regions. Namely, compared with healthy adults, schizophrenia patients would have a more center-left classification boundary in a “happy-sad” continuum of eye images and a more center-right classification boundary in a “happy-sad” continuum of mouth images.
Eighty-three schizophrenia patients who were in the phase of stabilization and convalescence were recruited for this study. Psychiatric diagnoses based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Psychopathological assessment was conducted by using the Positive and Negative Syndrome Scale (PANSS). According to previous studies, our criteria for being in the phase of stabilization and convalescence included two requirements [21,22]. First, no relevant PANSS item was greater than “mild” (no rating greater than 3) for both positive and negative symptoms. Second, there was no change in the types and dosages of drugs last month. In addition, included patients have certain communication and understanding abilities, therefore, they can cooperate with relevant inspections and complete the current research. Exclusion criteria included a history of head injury, co-morbid serious physical disease, substance abuse or alcohol abuse (except smoking), pregnancy or lactation, and chronic diseases of the nervous system.
Approval was obtained from the Shanxi Medical University Ethics Committee (no. 2018LL244). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Seventy-one healthy controls were recruited through advertisement, and were screened using a semi-structured interview to confirm the absence of psychiatric history or related illness. All schizophrenia patients as well as the healthy controls were right-handed and with normal or corrected-to-normal vision. Informed consents were signed by all participants.
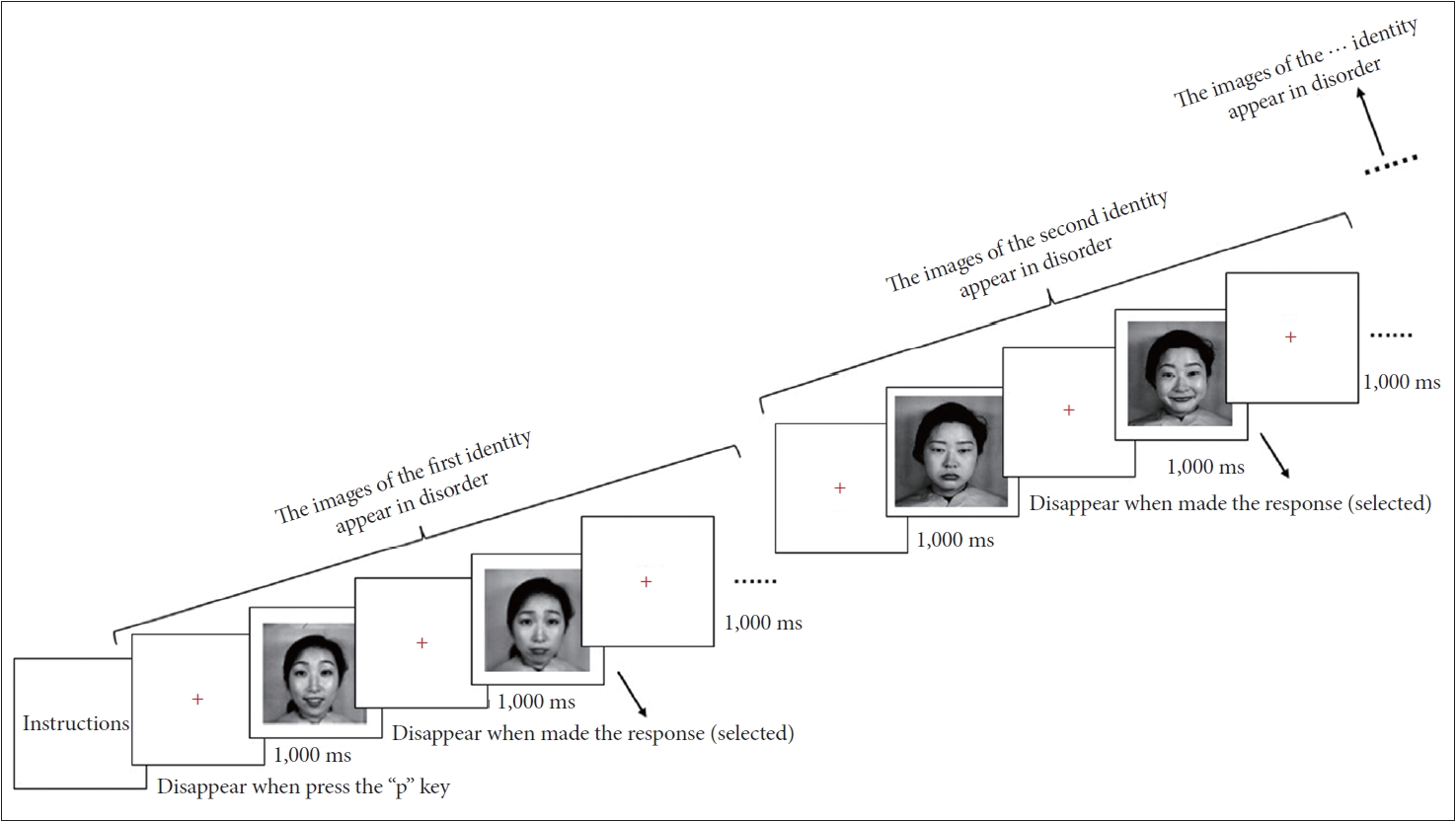
Standard happy and sad images of 10 Asian female identities were selected from the Japanese Female Facial Expression Database [23] as prototype pictures. The emotional intensity in “prototype happy” is defined to 1 and the same definition in “prototype sad” is 11. By using Abrosoft FantaMorph5 (Abrosoft, Lincoln, NE, USA) [24], each identity’s two prototype pictures (with 100% happy and 100% sad) were morphed to create a continuum of 11 facial images, and each intermediate image was transformed by a 10% increment with sad emotion signal increasing gradually. The “emotional intensity” in these morphed facial expressions between two prototype pictures was an integer value between 1 and 11 (see Figure 1). Thus, 10 identities generated 10 whole facial continuums, which consisted of 110 whole facial images. Each facial image contains a sad emotion with a different proportion. Details of the morphing technique were provided by Pollak and Kistler [25].
Next, these whole facial images were divided into upper and lower half images through a certain way described by Wegrzyn et al. [18] Using these upper and lower half facial images, other 20 continuums were generated (10 upper facial continuums and 10 lower facial continuums).
The experiment was performed using E-Prime 2.0 Professional Software (Psychology Software Tools, Pittsburgh, PA, USA) [26], and all facial images were exhibited by this psychological software.
In a quiet room, participants were seated before a 14-inch Acer Laptop screen with a resolution of 1,024×768 pixels. The distance from their eyes to the screen was approximately 57 cm. Prior to normal experiments, task instructions and emotional definitions were presented on the screen to help participants get to know experimental rules, and then participants pressed the ‘p’ key in a standard QWERTY keyboard to enter experiments. The formal experiment began with a red cross remaining 1 second in the center of the screen. Then 11 facial images of the first identity, with 256×256 pixels, were presented on the screen with out-of-order process. For each image, participants selected an emotion to describe the emotional facial expression appropriately by pressing the ‘f’ key for happy and ‘j’ key for sad. There was no time limit for responses, and the facial image remained in view until a response was made. The duration of responses approximates the timing of judgments in real circumstances. When participants made their choice, a trial finished. Between two trials, a red cross appeared in the center of the screen for 1 second. Before the formal experiment in each block, there was a practice session, in which 11 practice images were estimated. When the estimate of all ten identities was finished, a block was over. Participants have viewed 110 whole emotional facial images in all (11 facial images×10 identities).
In the other two blocks, participants were instructed to estimate emotions of the upper facial areas and lower facial areas by observing local facial images. At the end of each block, participants had a duration to rest. The order of the blocks was counterbalanced across participants. In summary, the formal experiment included three hundred and thirty trials (11 facial images×10 identities×3 face areas) distributed in 3 blocks (see Figure 2).
Previous studies have shown that logistic function models were fit for analyzing these kinds of dates, because S-shaped functions could appropriately fit the changing trend of emotional categorical perception [1,27]. As previous studies did [28], the logistic function y=a+(b-a)/(1+e-[(x-c)/d]), generated at the earliest by Pollak and Kistler [25], was theoretically more appropriate for fitting dates in current study. In this function, e represents exponential function, x is emotional intensity of sad emotion, y is the probability of identifying sad emotion, c represents the value of shift point. The value is equal to the emotional intensity of sad under the condition of y=50%, d is corresponding to the sensitivity, a and b represent the lower and the upper asymptotes, respectively. All parameters can be estimated through raw data from our experiments by using a nonlinear regression approach. Mathematically, the sensitivity is equal to (b-a)/4d. Because b-a approaches to the value 1, in current study, as previous studies did [25,28], we used 1/d as the approximate value of the sensitivity. It is the indicator of sensitivity to participants’ classification of emotional facial expressions
We employed a nonlinear regression approach to estimate the parametric value of c and d, then acquired the value of shift point (c), and the sensitivity of participants’ emotional categorical perceptions (1/d). We conducted a two-ways 2×3 repeated-measures analysis of variance of the shift point and sensitivity by using face areas as a within-subject factor and groups of participants as a between-subject factor. The dependent variables are shift point (c) and sensitivity (1/d).
Two groups completed the estimating task with three different face areas. After recruiting as candidates, 3 patients were excluded because their changing treatment condition made them unable to finish the whole experiment. On account of careless or random response, 9 patients’ data was invalid. In conclusion, 71 patients’ data were analyzed. We choose a moderate effect size (f=0.25) and a statistical power threshold of 0.8 (with p≤0.01) to calculate the sample size of our study. And according to this standard, we ensure that each group of 71 participants is enough to capture robust results of our experiment.
For the participants in the final analysis, all healthy controls match the schizophrenia group in gender, age, and education. Their clinical and demographic characteristics are summarized in Table 1. The descriptive statistical data of the shift points and sensitivities of three facial areas of schizophrenia patients and healthy controls are shown in Table 2.
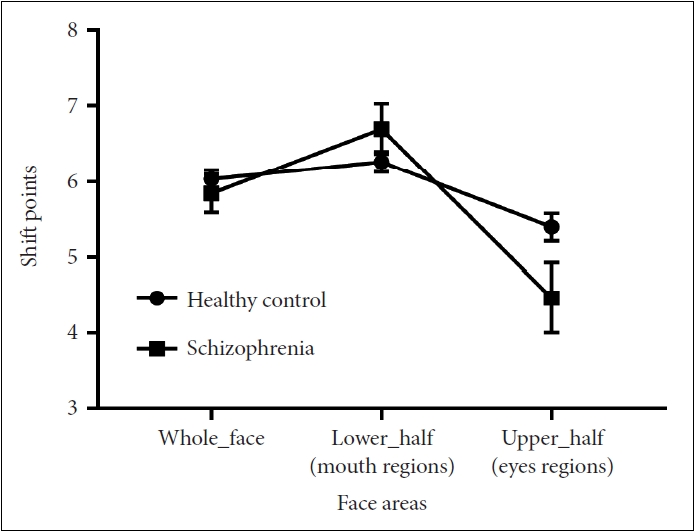
For the shift point, there was no main effect of group (F(1, 140)=2.43, p=0.12). A significant main effect of face areas indicated that for different face areas, shift point was distinct despite groups (F(1.63, 227.76)=99.26, p<0.001, η2p=0.415). A significant group-by-face area interaction forecasted that shift points among face areas are different for two groups (F(1.63, 227.76)=18.72, p<0.001, η2p=0.118) (see Table 3 and Figure 3).
Next, a simple effect analysis of groups showed a significant difference between two groups in the condition of upper (p<0.001) and lower (p=0.015) facial expressions, but not in the whole facial expressions (p=0.182). A simple effect analysis of face areas revealed that significant differences exist in every pairwise comparisons for schizophrenia patients (for all p<0.001). However, such significant results existed in only the comparison of upper to lower facial expressions (p<0.001) and upper to whole facial expressions (p=0.002) for the healthy control.
For the sensitivity, a significant main effect of groups indicated that schizophrenia patients and healthy controls’ sensitivity about emotional facial expressions was different (F(1, 140)=17.70, p<0.001, η2p=0.112). A significant main effect of face areas indicated that participants had dissimilar sensitivity among different face areas (F(2, 280)=7.20, p=0.001, η2p=0.049). There was no significant group-by-face area interaction (F(2, 280)=1.10, p=0.335) (see Table 3 and Figure 4). Then, LSD test was carried out for different groups, the result showed that in all face areas, sensitivity of two groups had significant difference (p<0.001). Specifically, the sensitivity of schizophrenia patients was significantly lower than that of healthy controls in all face areas.
The logistic data response curves in three face areas between schizophrenia patients and healthy controls were illustrated based on their raw data (see Figure 5).
This study confirmed schizophrenia patients’ integrated deficit of signals on facial area by analyzing schizophrenia patients’ patterns of emotional categorical perception under different facial areas conditions.
Schizophrenia patients’ shift point is the same as that of healthy controls under the condition of whole facial areas, which is inconsistent with previous studies [29]. Potential reasons may be due to the different recovery levels of the patients. In previous studies, participants were inpatients with schizophrenia, while the current study uses patients in the phase of stabilization and convalescence, who have better physical and mental states when operating tasks. Thus, they presented more similar shift points with healthy controls, which potentially implied the improvement of emotional functions for schizophrenia patients in the phase. It also indicated that for schizophrenia patients with relatively slight pathological characteristics, their estimated pattern of ambiguous emotional facial expressions is similar to healthy people in the daily life. The phenomenon is consistent with the study of Grave et al. [30] They have proved that although schizophrenia patients have an overall slower access to visual awareness of facial expressions, the implicit perception of emotional faces is intact.
Compared to healthy controls, schizophrenia patients significantly prefer estimating ambiguous emotional facial expressions as happy expressions (namely, a more center-right classification boundary) when they process lower faces, whereas significantly prefer estimating ambiguous emotional facial expressions as sad expressions (namely, a more center-left classification boundary) when they process upper faces. It indicates that the pattern of emotional categorical perception for schizophrenia patients is significantly different from that of healthy controls when they process signals on the local facial areas. Compared to healthy people, schizophrenia patients have a significantly separate classification pattern in processing emotional signals between the eyes and mouth regions. Combining the similar shift points for the two types of populations under the condition of processing signals on whole faces, the different sensitivity between the two types of populations should be derived from the significantly separated classification pattern in schizophrenia patients and further attributed to their integrated deficit of signals on facial areas. In addition, the reason for the significantly separated classification pattern in schizophrenia patients under the condition of processing local facial areas could be explained by the socioemotional selectivity theory.
Socioemotional selectivity theory advocates that individuals’ attention preference for emotional facial expressions corresponds to their levels of social development [21-33]. Previous studies have found that people with relatively low socialization levels, such as children or mentally disabled people, were apt to pay attention to people’s mouths. [33]. Ample evidence has shown degraded socialization level of schizophrenia patients [34]. Hence, schizophrenia patients are prone to having preferences for mouth region. Moreover, because the mouth region contains more traces of happy emotions [35-38], they are habituated to acquire more signals of happiness from mouth regions, and prefer perceiving ambiguous emotional facial expressions as happiness when they observe signals from mouth regions.
Simultaneously, socioemotional selectivity theory also supposed that attention preference for emotional facial expressions is closely related to individual’s living environments [32]. Because of clinical symptoms, schizophrenia patients often feel dangerous about their surroundings. It prompts them to notice negative emotional facial expressions, such as sad expressions. Moreover, the eye region is considered to contain more signals of emotions with negative valence, such as sadness [39]. It contributes to the phenomenon that schizophrenia patients perceive ambiguous emotional facial expressions as having sad emotions when they observe signals from eye regions. This phenomenon does not conflict with schizophrenia patients’ preferences to pay more attention to mouth regions and to reduce observation of eye regions, because our experiment forces schizophrenia patients to process solo eye and mouth regions respectively in different blocks.
To overcome the conflict of the significantly separated classification pattern during processing the ambiguous emotional facial expressions on a whole face, schizophrenia patients require more cognitive resources. However, previous studies have proved that the deficit of cognitive resources is a key pathological feature of schizophrenia patients [40-43]. Therefore, schizophrenia patients have to take more time to process ambiguous emotional facial expressions in daily life, and they still have difficult to estimate these expressions as quickly and accurately as healthy people. As a result, the sensitivity to the classification of emotional facial expressions is relatively low.
The result of the sensitivity to the classification of emotional facial expressions is fitting for the above explanations. In all facial area conditions, the sensitivity of schizophrenia patients was significantly lower than that of healthy control. The fact revealed that schizophrenia patients were more insensitive to classifying facial expressions with positive and negative emotions, which was consistent with previous studies [1,44]. This might cause the inability of schizophrenia patients to detect changes of people’s emotions in social communications in time, leading to lags in response in daily life to some degree.
To sum up, the abovementioned discussions have proved that the deficit of categorical perception of emotional facial expressions in schizophrenia patients is derived from an integrated deficit of signals on facial area. Specifically, for schizophrenia patients in the phase of stabilization and convalescence, their abilities of classification of emotional facial expressions in the whole face are relatively intact. It indicates that the relatively low sensitivity of emotional facial expressions did not result from an emotion-specific deficit. Meanwhile, schizophrenia patients perceive more negative emotions when they observe ambiguous facial expressions in eye regions, and perceive more positive emotions when they observe ambiguous facial expressions in mouth regions. It reflects a significantly separate classification pattern in processing emotional signals between the eyes and mouth regions, and leads to a conflict in the processing of estimating ambiguous emotional facial expressions on a whole face. Schizophrenia patients cannot mobilize sufficient cognitive resources to overcome this conflict, and fail to integrate ambiguous even contradictory emotional signals from different facial areas. Consequently, the sensitivity to the classification of emotional facial expressions becomes relatively low. Namely, an integrated deficit is the reason for abnormal categorical perception of emotional facial expressions. At least for schizophrenia patients in the phase of stabilization and convalescence. Further studies are required to investigate this debate by using some physiological technologies, such as eye movement approaches.
Notes
Availability of Data and Material
The data that support the findings of this study are available from the author upon reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jian Zhang. Data curation: Yunzhen Xue. Formal analysis: Ruomin Wang. Investigation: Ruomin Wang. Methodology: Jian Zhang. Software: Jian Zhang. Validation: Yunzhen Xue. Writing—original draft: Jian Zhang. Writing—review & editing: Yunzhen Xue.
Funding Statement
This study was funded by the National Social Science Foundation of China (grant number 19BSH168).
ACKNOWLEDGEMENTS
We are grateful to the patients who participated in this study and the doctors who worked together.
Figure 5.
Response curves about participants and facial areas. A: Response curves about participants. B: Response curves about facial areas. The slope of the response curves represents the sensitivity to the classification of emotional facial expressions.
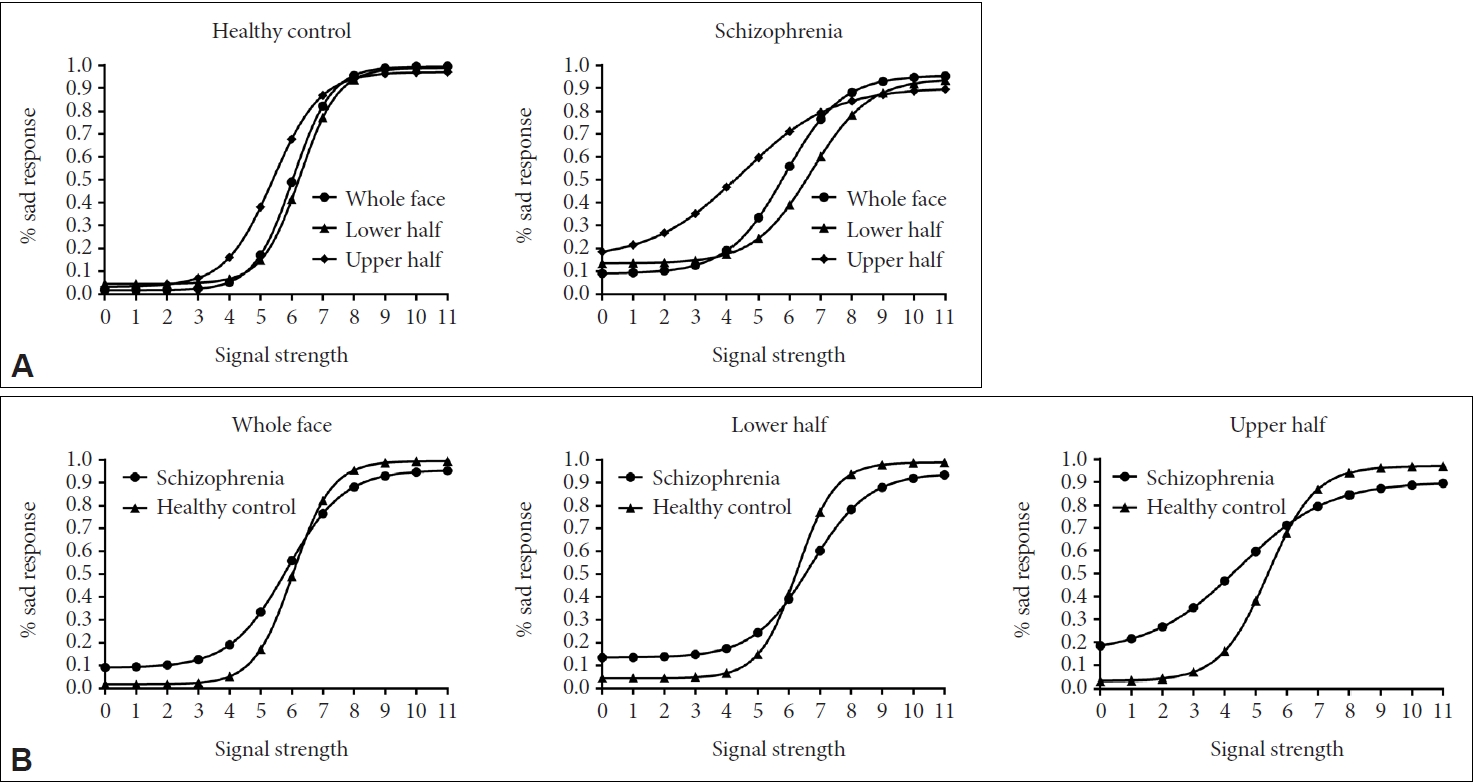
Table 1.
Demographic characteristics of schizophrenia patients and healthy controls
Table 2.
Shift points and sensitivities of three face areas of schizophrenia patients and healthy controls
REFERENCES
1. Kee KS, Horan WP, Wynn JK, Mintz J, Green MF. An analysis of categorical perception of facial emotion in schizophrenia. Schizophr Res 2006;87:228-237.


2. Mancuso M, Magnani N, Cantagallo A, Rossi G, Capitani D, Galletti V, et al. Emotion recognition impairment in traumatic brain injury compared with schizophrenia spectrum: similar deficits with different origins. J Nerv Ment Dis 2015;203:87-95.

3. Marosi C, Fodor Z, Csukly G. From basic perception deficits to facial affect recognition impairments in schizophrenia. Sci Rep 2019;9:8958




4. Mote J, Kring AM. Facial emotion perception in schizophrenia: does sex matter? World J Psychiatry 2016;6:257-268.



5. Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull 2013;39:979-992.


6. Wynn JK, Jahshan C, Altshuler LL, Glahn DC, Green MF. Event-related potential examination of facial affect processing in bipolar disorder and schizophrenia. Psychol Med 2013;43:109-117.


7. Zhu CY, Lee TM, Li XS, Jing SC, Wang YG, Wang K. Impairments of social cues recognition and social functioning in Chinese people with schizophrenia. Psychiatry Clin Neurosci 2007;61:149-158.


8. Berger P, Bitsch F, Jakobi B, Nagels A, Straube B, Falkenberg I. Cognitive and emotional empathy in patients with schizophrenia spectrum disorders: a replication and extension study. Psychiatry Res 2019;276:56-59.


9. Kuis DJ, van de Giessen T, de Jong S, Sportel BE, Boonstra N, van Donkersgoed R, et al. Empathy and its relationship with social functioning in individuals at ultra-high risk for psychosis. Front Psychiatry 2021;12:730092



10. Lehmann A, Bahçesular K, Brockmann EM, Biederbick SE, Dziobek I, Gallinat J, et al. Subjective experience of emotions and emotional empathy in paranoid schizophrenia. Psychiatry Res 2014;220:825-833.


11. St Pourcain B, Robinson EB, Anttila V, Sullivan BB, Maller J, Golding J, et al. ASD and schizophrenia show distinct developmental profiles in common genetic overlap with population-based social communication difficulties. Mol Psychiatry 2018;23:263-270.



12. Tordjman S, Celume MP, Denis L, Motillon T, Keromnes G. Reframing schizophrenia and autism as bodily self-consciousness disorders leading to a deficit of theory of mind and empathy with social communication impairments. Neurosci Biobehav Rev 2019;103:401-413.


13. Silver H, Shlomo N, Turner T, Gur RC. Perception of happy and sad facial expressions in chronic schizophrenia: evidence for two evaluative systems. Schizophr Res 2002;55:171-177.


14. Bediou B, Krolak-Salmon P, Saoud M, Henaff MA, Burt M, Dalery J, et al. Facial expression and sex recognition in schizophrenia and depression. Can J Psychiatry 2005;50:525-533.



15. Yildirim E, Yalinçetin B, Sevilmiş Ş, Kutay Ö, Alptekin K. Is there any relation between impaired emotion perception and thought disorder in schizophrenia? Noro Psikiyatr Ars 2018;55:118-122.



16. Craig BM, Chen NTM, Lipp OV. Featural vs. holistic processing and visual sampling in the influence of social category cues on emotion recognition. Cogn Emot 2022;36:855-875.


17. Li Y, Tian Y, Wu Q, Leng H, Jiang Z, Yang Y. Effects of expression on social perceptions of faces. Advances in Psychological Science 2021;29:1022-1029.


18. Wegrzyn M, Bruckhaus I, Kissler J. Categorical perception of fear and anger expressions in whole, masked and composite faces. PLoS One 2015;10:e0134790



20. Harnad S. Psychophysical and cognitive aspects of categorical perception: a critical overview. Cambridge: Cambridge University Press; 1987.
21. Whitehorn D, Brown J, Richard J, Rui Q, Kopala L. Multiple dimensions of recovery in early psychosis. Int Rev Psychiatry 2002;14:273-283.

22. Kukla M, Lysaker PH, Roe D. Strong subjective recovery as a protective factor against the effects of positive symptoms on quality of life outcomes in schizophrenia. Compr Psychiatry 2014;55:1363-1368.


23. Lyons MJ, Akamatsu S, Kamachi M, Gyoba J, Budynek J. The Japanese female facial expression (JAFFE) database. In: Proceedings of the Third IEEE International Conference on Automatic Face & Gesture Recognition; 1998 Apr 14-16; Nara, Japan. IEEE. 1998;14-16.
25. Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proc Natl Acad Sci U S A 2002;99:9072-9076.



26. Documentation articles: E-prime. Available at: https://support.pstnet.com/hc/en-us/categories/204686967-EPrime. Accessed December 5, 2022.
27. Tsui CF, Huang J, Lui SS, Au AC, Leung MM, Cheung EF, et al. Facial emotion perception abnormality in patients with early schizophrenia. Schizophr Res 2013;147:230-235.


28. Qiu F, Han M, Zhai Y, Jia S. Categorical perception of facial expressions in individuals with non-clinical social anxiety. J Behav Ther Exp Psychiatry 2018;58:78-85.


29. Huang J, Chan RC, Gollan JK, Liu W, Ma Z, Li Z, et al. Perceptual bias of patients with schizophrenia in morphed facial expression. Psychiatry Res 2011;185:60-65.


30. Grave J, Madeira N, Martins MJ, Silva S, Korb S, Soares SC. Slower access to visual awareness but otherwise intact implicit perception of emotional faces in schizophrenia-spectrum disorders. Conscious Cogn 2021;93:103165


31. Carstensen LL, Fung HH, Charles ST. Socioemotional selectivity theory and the regulation of emotion in the second half of life. Motiv Emot 2003;27:103-123.
32. Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously. A theory of socioemotional selectivity. Am Psychol 1999;54:165-181.


33. Gu L, Bai X. Visual preference of facial expressions in children and adults: evidence from eye movements. Journal of Psychological Science 2014;37:101-105.
34. Zhu Y, Fang J, Zhang B, Luo YZ, Zhao W, Wang X. Functional neuroimaging of social cognition in schizophrenia. Chinese Journal of Clinical Psychology 2014;22:232-239.
35. Calvo MG, Fernández-Martín A. Can the eyes reveal a person’s emotions? Biasing role of the mouth expression. Motiv Emot 2013;37:202-211.


36. Calvo MG, Fernández-Martín A, Nummenmaa L. A smile biases the recognition of eye expressions: configural projection from a salient mouth. Q J Exp Psychol (Hove) 2013;66:1159-1181.



37. Calvo MG, Fernández-Martín A, Nummenmaa L. Facial expression recognition in peripheral versus central vision: role of the eyes and the mouth. Psychol Res 2014;78:180-195.



38. Nummenmaa L, Calvo MG. Dissociation between recognition and detection advantage for facial expressions: a meta-analysis. Emotion 2015;15:243-256.


39. Tan JW, Walter S, Scheck A, Hrabal D, Hoffmann H, Kessler H, et al. Repeatability of facial electromyography (EMG) activity over corrugator supercilii and zygomaticus major on differentiating various emotions. J Ambient Intell Human Comput 2012;3:3-10.


41. Luck SJ, Hahn B, Leonard CJ, Gold JM. The hyperfocusing hypothesis: a new account of cognitive dysfunction in schizophrenia. Schizophr Bull 2019;45:991-1000.




42. Sarin F, Wallin L. Cognitive model and cognitive behavior therapy for schizophrenia: an overview. Nord J Psychiatry 2014;68:145-153.