 |
 |
- Search
| Psychiatry Investig > Volume 20(2); 2023 > Article |
|
Abstract
Objective
The study investigated cognitive performance and brain function between treatment-resistant depression (TRD) and non-TRD patients to find potential neurobiological markers associated with refractoriness in depression patients.
Methods
Fourteen TRD patients, 26 non-TRD patients and 23 healthy controls (HC) were included in the present study. The neural function of prefrontal cortex (PFC) and cognitive performance among the three group were examined using near-infrared spectroscopy (NIRS) during verbal fluency task (VFT).
Results
Both TRD and non-TRD groups exhibited significantly worse VFT performance and lower activation of oxygenated hemoglobin (oxy-Hb) changes in the bilateral dorsolateral PFC (DLPFC) compared to the HC group. Within the TRD and non-TRD groups, VFT performance was no significant difference, but activation of oxy-Hb changes in dorsomedial PFC (DMPFC) in TRD patients was significantly lower than non-TRD patients. In addition, activation of oxy-Hb changes in right DLPFC were negatively correlated with the severity of depressive symptoms in depression patients.
Even though applied sequential trials of antidepressant treatments, there are 20%-30% depression patients fail to respond to standard treatments [1,2]. The definition of treatmentresistant depression (TRD) is considered as fail to respond to at least two different antidepressants prescribed at adequate dosages and durations or do not achieve clinical remission [3]. TRD may result in poorer cognitive performance [4], worse psychosocial functioning and higher suicidality [5]. Studying the potential neurobiological markers of TRD may help better recognized patients who are treatment resistance and develop novel effective therapeutics.
Nowadays, there are lots of neuroimaging researches studied major depressive disorder (MDD). However, studies about the structure, blood flow and tissue metabolism of the brain in TRD are fewer. Among the existing researches, the results of are inconsistent or contrary. For example, Duhameau et al. [6] used arterial spin labelling perfusion MRI to find that six TRD patients exhibited hyperperfusion in the left dorsomedial prefrontal cortex (DMPFC) and anterior cingulate cortex. Yamamura et al. [7] also discovered TRD patients showed increased spontaneous neural activity in inferior frontal gyrus using resting-state functional magnetic resonance imaging. While recently Li et al. [8] showed that prefrontal cortex (PFC) hypometabolism paly a key role in the TRD neuropathology with positron emission tomography scans. These inconsistent results are unsurprising, as whether variable such as antidepressants and past depressive episodes affect on prefrontal activation is unknown. Even so, these studies provide the clues that the PFC function alteration may be involved in the possible mechanisms in TRD patients. Indirect evidence supported dysfunction of PFC is that by taking repetitive transcranial magnetic stimulation of the dorsolateral PFC (DLPFC), the symptoms of TRD patients have showed to improve [9-11].
PFC not only can exert top-down control over limbic system as a regulatory mechanism for depression [12], but also is a key neural basis part of cognitive function [13]. Li et al. [8] proved attentional performance were significant difference between TRD patients and non-TRD patients. Unfortunately, the patients sample in this study was just grouped according to if patients have history of medication resistance. It should be worth noticed that more and more studies reported MDD lead poorer cognition without symptomatic remission [4,5]. Therefore, we cannot conclude that the cognitive function of TRD patients were more severe than non-TRD. In addition, which abnormalities of PFC related to diminished cognitive functioning is worth studying.
Near-infrared spectroscopy (NIRS) is a noninvasive optical neuroimaging technique that can measure oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) concentrations in brain tissue which reflected the neural activity [14]. Due to the advantage of relatively nonconstraining, low consumption, portable and repetitive operation, it has be widely used to investigate brain function during cognitive task in several psychiatric disorders especially depressive disorder [15]. However, the specific region which impair depressive symptoms still remain unclear. For example, Akiyama et al. [16] used NIRS found that left frontal activations is significantly smaller during verbal fluency task (VFT) in MDD patients compared with health controls. Whereas most research showed bilateral prefrontal significantly lower activation [17-19]. In addition, recent study suggest remitted depressive patient showed left frontotemporal lower hemodynamic activations [20]. One major reason that may explain the inconsistency is that samples contains clinical subtypes such as refractory depression. To better research the different neural activity between TRD patients and non-TRD patients, this study use NIRS to examine the difference hemodynamic response in PFC area during VFT. As the widely used task in neuropsychological cognitive assessment, VFT has been proved to detect PFC activation in health people and mental disorder patients combined with NIRS and can assess executive control functions by self-monitor [21,22]. In addition, it was the most commonly used task in cognitive neuroimaging study. Therefore, it was selected as the cognitive task in this study.
The aim of this study was to evaluate cognitive performance and prefrontal hemodynamic response between TRD and non-TRD patients use NIRS during VFT. It was hypothesized that impaired performance and decreased prefrontal activation would be observed, furthermore, prefrontal activation pattern associated with cognition would differ in TRD and non-TRD patients.
The study was approved by the ethics review committee of Yu Quan Hospital, and written informed consent was obtained from all participants prior to the commencement of the study. Patients with MDD were recruited from inpatient departments of Yu Quan Hospital from September 2013 to August 2016. The diagnoses of all patients were established by the Structured Clinical Interview according to the DSM-IVTR (SCID). Exclusion criteria include: 1) age less than 18 or over 60 years; 2) left-handed which is evaluated by Edinburgh handedness inventory; 3) major physical illnesses such as malignant tumor, chronic renal failure, severe heart disease, and severe respiratory disease; 4) other psychiatric disorders and neurological disorders; 5) substance abuse; 6) took medication within the 3-month period prior to the study; and 7) received electroconvulsive therapy. Finally, 40 patients were include in the present study. Twenty-three right-handed healthy controls (HC) were recruited from the local community by advertisements. Age, gender and educational level were matched between MDD groups and HC. The control subjects were screened using the non-patient edition of the SCID to confirm the lifetime absence of a history of psychiatric or neurological disorders and were interviewed to exclude family history of psychiatric illness.
Before started antidepressant treatment, each participants underwent NIRS imaging. Antidepressants were empirically prescribed according to the clinical judgment of the treating psychiatrist. Three classes of antidepressants include tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and selective serotonin reuptake inhibitors were used.
Treatment resistant is defined as a poor response to at least two different classes of antidepressant trials with each trail in adequate dosages and duration (6 weeks) [23]. Specifically, after antidepressant treatment, patients who have less than 50% Hamilton Depression Rating Scale (HAMD, 24-item) [24] score reduction were considered as TRD patients and more than 50% were considered as non-TRD patients.
Present task procedure was similar to previous study [25,26]. We took semantic category versions of the VFT to stimulate prefrontal activation which is reflected prefrontal hemodynamic responses. Simultaneously measured oxy-Hb changes during VFT. The procedure was four block (vegetables, domestic applications, four-legged animals, and fruits) with each block include 15 second pre-task baseline, 30 second task and 15 second pro-task baseline. Each participants were sat comfortably in the front of screen presented task with a distance of 50 cm. During pre-task and pro-task period, they were asked to stare at the symbol of “+” on screen quietly, while during the task period, they were asked to generate as many Chinese words as possible that belonged to the designated species. The total numbers of correct words generated during VFT period were represented the participants’cognitive performance scores. In addition, we took index of the accuracy (%) of the correct words to evaluate behavioral performance.
Forty-five channels NIRS machine (FOIRE-3000, Shimadzu Corporation, Kyoto, Japan) was used to measures oxy-Hb and deoxy-Hb during cognitive task. The 14 pairs of probe arrangement used 7×4 installation modes with each channel (ch) contain a pair of emitter and detector probe at the distance of 3 cm. The most inferior probes were positioned along the Fp1-Fp2 line according to the International 10-20 system of electroencephalogram electrode placement [27]. With the probe arrangement, NIRS can measure oxy-Hb and deoxy-Hb concentration changes of PFC. The time resolution was set 0.1 sec. Before export data, baseline corrections and filtered were used to reduce artifact motion.
We used one way analysis of variance (ANOVA) to analyze continuous variables include demographic characteristics (age and education level), clinical variables (illness duration, past depressive episodes, HAMD score) and VFT performance (the correct number of items, accuracy) among the three groups. As to categorical variables (gender) among groups, we used contingency table analysis. ANOVA was also used to analyze oxy-Hb changes differences among the three groups.
The value of oxy-Hb changes (calculated by subtracting the mean oxy-Hb of the task period from that mean oxy-Hb of the pre-task period), as a much reliable statistical indicator [28,29], stronger correlation with blood oxygenation level-dependent signal measured by functional MRI [30] and better signal to noise ratio than deoxy-Hb change [31], is selected to directly reflect regional cerebral blood flow changes and cognitive activation. For further post hoc test, we took Bonferroni test. Homogeneity of variance was tested to compare the distribution shape of the different groups. Benjamini-Hochberg’s procedure was adopted for multiple comparisons analyses of 45-channel testing correction. The q value of the maximum false discovery rate (FDR) was set at 0.2 so that the false positive rate was no higher 20% on average (FDR-corrected).
To examine the relationships between oxy-Hb changes in each channel and clinical variables, Spearman’s rank correlation coefficients were calculated for TRD patients and non-TRD patients.
All statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (SPSS version 22.0; IBM Corp., Armonk, NY, USA).
As shown in Table 1, the age, gender, and education level were not significant different among TRD, non-TRD and HC groups. Between TRD and non-TRD groups, illness duration and severity of depressive symptoms before treatment as assessed with HAMD showed not significant differences, but HAMD score after treatment were significant differences. In addition, HAMD score before treatment were significant differences between TRD and HC groups and between non-TRD and HC groups. However, HAMD score after treatment were not significant different between non-TRD and HC groups, but still exhibit statistically significant differences between TRD and HC groups.
The VFT performance of the three groups was shown in Table 1. As it presented, the correct number of items among the three groups was statistically significant differences in the four categories. When compared two of the three groups, both the TRD and non-TRD groups generated a significantly less number of items in all categories compared to the HC group; however, no significant differences between the TRD group and non-RD group in all categories. The accuracy among the three groups were not significantly different.
Oxy-Hb change were homogeneous in 34 channels (p>0.05), except channels 7, 10, 19, 20, 26, 29, 31, 35, 37, 42, 43. In the 32 channels, ANOVA revealed significant differences among groups in channels 14, 21-23, 25, 30 (FDR-corrected p<0.2) (Figure 1). When use Bonferroni to conduct the post hoc analysis, non-TRD patients exhibited significantly lower oxy-Hb activation than HC groups in channels 21 (FDR-corrected p<0.2) (Figure 2A). TRD patients exhibited significantly lower oxy-Hb activation than HC groups in channels 14, 21- 23, 25, 30 (FDR-corrected p<0.2) (Figure 2B). Both patients exhibited a reduced activation in channels 21 when compared with HC groups. In direct comparison within TRD and non-TRD groups, the mean oxy-Hb changes in TRD patients were significantly smaller than those in non-TRD patients in channel 23 and 30 (FDR-corrected p<0.2) (Figure 2C and D).
In TRD and non-TRD group, a significant negative correlation between mean oxy-Hb changes and HAMD scores in channel 21 (FDR-corrected p<0.2) (Figure 3), which approximately located in right DLPFC. A significant positive correlation between mean oxy-Hb changes and the reduction rate of HAMD scores in channel 21 (FDR-corrected p<0.2) (Figure 4), which approximately located in DMPFC. Furthermore, no significant correlation was observed between the oxy-Hb changes and illness duration (FDR-corrected p>0.2) in tested channels in TRD and non-TRD group.
This study used NIRS imaging to measure the hemodynamic response associated with cognitive activation in TRD patients and non-TRD patients. The comparison of VFT performance and PFC activation among TRD, non-TRD and HC group showed that both TRD and non-TRD patients exhibited significantly worse performance and smaller oxy-Hb activation in DLPFC. Within TRD and non-TRD groups, there were no significant difference in VFT performance, however, the activation of oxy-Hb changes in TRD patients was significantly smaller than non-TRD patients in DMPFC. Furthermore, depressive symptoms severity as assessed by the HAMD showed significant negative correlation with right DLPFC in TRD patients and non-TRD patients.
To our knowledge, this study is the first report using NIRS imaging to evaluate cognitive performance and prefrontal hemodynamic response in the PFC area between TRD and non-TRD patients during VFT. NIRS may assist to understand the neural basis of treatment resistant in MDD patients.
VFT is a kind of neuropsychological test to assess executive functions [32]. Compared with HC group, both TRD and non-TRD patients generated significantly fewer correct words in the four categories. This finding demonstrate the executive functions of MDD patients were impaired. Producted less number of correct words during VFT may imply that deterioration of semantic storage and dysfunction of frontal cortex which related to the ability to retrieve a series of nouns [33]. The result is consistent with previous studies which proved MDD patients exhibited poorer performance of executive function than normal control [34-37]. As far as we know, there are studies reported no impairment of VFT performance between MDD patients and normal control [18,38-40]. In these studies, the different time setting may explain the discrepancies. In our version of VFT, the time setting was four categories words within 4 minutes and 30 seconds for each category task period. Using the version of VFT with the same four categories words but increase time during silence, our lab showed that there were no significant differ between MDD patients and normal controls [18]. The reason may be that the patients get relax with extended time and the subject can keep generating words regularly without the influence of task period.
However, TRD and non-TRD patients performed similar. This finding may suggest that the extent of executive function impairment were similar and the trait of treatment resistant have a minimal impact on this kind of cognitive performance in keep generating words regularly. The result is differ with previous study which suggest attentional deficits in TRD patients [8] and may represent different aspect cognitive function abnormal in TRD patients, but need more research to support the result.
In the present study, we found TRD and non-TRD patients showed smaller oxy-Hb activation in DLPFC prefrontal compared with HC group. This finding is in accord with previous functional neuroimaging studies which examined activation of depression patients’ prefrontal regions during cognitive tasks [39-41]. Hypoactivity in the prefrontal area during VFT task period has been suggested that depression patients cannot able to obtain a corresponding increase oxygen in blood supply to compensate for consumed, which is crucial for proper neuronal activity. DLPFC plays an important role in regulating emotions [42] and cognition [43,44]. Thus dysfunction of the area lead to insufficiencies in emotion regulationand cognitive aspect in depression patients. Furthermore, the severity of depression of TRD and non-TRD patients had a negative correlation with oxy-Hb changes in the region of right DLPFC. This may suggest neural vulnerability in terms of the severity of depression symptoms. The lower oxy-Hb activation in the right DLPFC trend to increase gradually in the severity of depression symptoms. The dysfunction of DLPFC may return to normal after antidepressants prescribed at adequate dosages and durations. That is to say, with the depression symptoms alleviated, oxy-Hb changes in the right DLPFC will be increased.
We observed a lower oxy-Hb activation mainly located in DLPFC and DMPFC (ch 14, 21-23, 25, 30) in the TRD patients compared with HC. While compared with non-TRD patients, TRD patients exhibited a lower oxy-Hb activation in channel 23 and 30 of the DMPFC. In addition, the reduction rate of HAMD scores of TRD and non-TRD patients had a positive correlation with oxy-Hb changes in the region of DMPFC. In this study, patients were in first episode and medicine free. Then this result may present a profile that biological factors paly essential role in TRD patients.
Previous studies have shown abnormal connectivity of medial prefrontal regions and thalamus is related to refractoriness in depression patients [45]. The lower activation of oxy-Hb in medial prefrontal regions predict poor response to SSRI in depression patients [46]. Samson et al. [47] showed greater activation in DMPFC response better to pharmacological treatment. Disrupted connectivity of medial PFC was continued exist in depression patients after 12 weeks of antidepressant treatment [48]. All this study emphasize the importance role of medial prefrontal regions in the refractory depression patients.
As a central role in affect regulation, DMPFC has been identified as dorsal nexus—a unique region of cortical network which converge cognitive control, emotion regulation and self-reflection [49]. Recent studies have shown that reduced activation in the middle frontal gyrus is an indicator of trait-related brain abnormalities in MDD patients [50]. So we speculate that abnormal activation in DMPFC area may be the pathological basis of refractoriness and may characterize a subgroup of depression people associate with refractoriness.
There are some limitations should be pointed out in our studies. Firstly, the study data is cross-sectional, whether abnormal activation prefrontal area change after antidepressant need further data in longitudinal studies. Secondly, the refractoriness of participating in present study were confirmed by the definition—a poor response to at least two different classes of antidepressant trials with each trail in adequate dosages and duration (6 weeks), not according to if patients have history of medication resistance. Thirdly, we didn’t exclude the MDD patients with anxiety.
In conclusion, TRD patients and non-TRD patients exhibited significantly worse performance and smaller oxy-Hb activation in DLPFC compared with HC subjects. Within TRD and non-TRD groups, there were no significant difference in VFT performance. However, the activation of oxy-Hb changes in TRD patients was significantly smaller than non-TRD patients in the bilateral DMPFC. This preliminary exploratory study suggest different prefrontal activation patterns between TRD patients and non-TRD patients. fNIRS may serve as a useful tool for distinguishing TRD patients from depressed patients by measuring prefrontal activation patterns patterns. Furthermore, depressive symptoms severity as assessed by the HAMD showed significant negative correlation with right DLPFC in TRD patients and non-TRD patients.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jing-Jing Sun, Po-Zi Liu. Data curation: Chen-Yu Shen. Formal analysis: Jing-Jing Sun. Investigation: Xiao-Min Liu. Project administration: Po-Zi Liu. Supervision: Chen-Yu Shen. Validation: Xiao-Min Liu. Writing—original draft: Jing-Jing Sun. Writing—review & editing: Chen-Yu Shen, Xiao-Min Liu, Po-Zi Liu.
Funding Statement
This study was supported by the Independent Scientific Research Program of Tsinghua University (No 548105001).
Figure 1.
p-value significant map for oxy-Hb changes comparison among TRD patients, non-TRD patients and HC during the VFT. Channels in colored showed significantly differ oxy-Hb changes among in TRD patients, non-TRD patients and HC during the VFT (FDR-corrected p<0.2). Channels in grey showed FDR-corrected p>0.2. TRD, treatment-resistant depression; HC, health controls; oxy-Hb, oxygenated hemo-globin; VFT, verbal fluency task; FDR, false discovery rate.
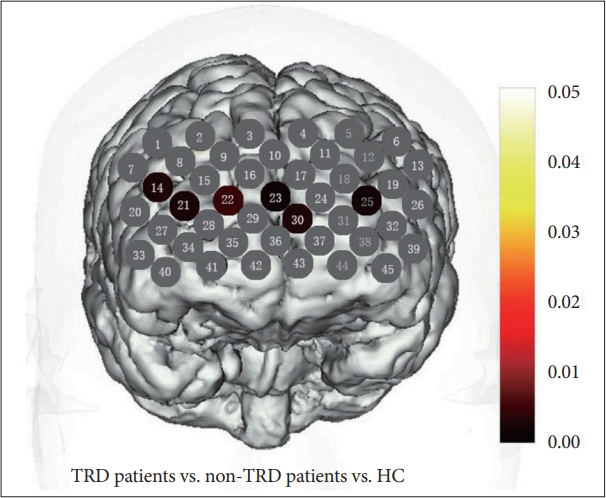
Figure 2.
p-value significant map for oxy-Hb changes for post hoc test comparison among TRD patients, non-TRD patients and HC during the VFT. A: Channels in colored showed significantly lower oxy-Hb changes in non-TRD patients compared with HC (FDR-corrected p<0.2). B: Channels in colored showed significantly lower oxy-Hb changes in TRD patients compared with HC (FDR-corrected p<0.2). C: Channels in colored showed significantly lower oxy-Hb changes in TRD patients compared with non-TRD patients (FDR-corrected p<0.2). D: Histogram of the average oxy-Hb changes in 6 channels among TRD patients, non-TRD patients, and HC during the VFT. TRD, treatment-resistant depression; HC, health controls; oxy-Hb, oxygenated hemo-globin; VFT, verbal fluency task; FDR, false discovery rate.

Figure 3.
Correlation with depression symptom severity in oxy-Hb changes during the VFT. The scatter plot illustrates a significant negatively correlation between mean oxy-Hb changes and HAMD scores in channel 21 (FDR-corrected p<0.2). oxy-Hb, oxygenated hemo-globin; HAMD, Hamilton Rating Scale for Depression; VFT, verbal fluency task; FDR, false discovery rate.

Figure 4.
Correlation between the reduction rate of HAMD scores and oxy-Hb changes. The scatter plot illustrates a significant positively correlation between mean oxy-Hb changes and reduction rate of HAMD scores in channel 30 (FDR-corrected p<0.2). oxy-Hb, oxygenated hemo-globin; HAMD, Hamilton Rating Scale for Depression; FDR, false discovery rate.
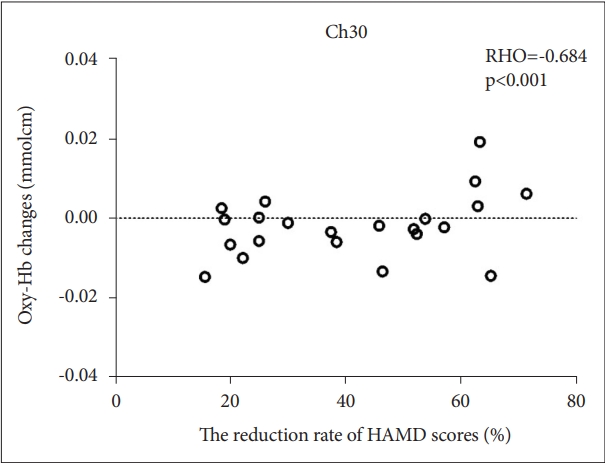
Table 1.
Characteristics of subjects
| Demographics | Non-TRD | TRD | HC | Significance (non-TRD, TRD, HC) | Significance (non-TRD vs. HC) | Significance (TRD vs. HC) | Significance (non-TRD vs. TRD) | |
|---|---|---|---|---|---|---|---|---|
| Age (yr) | 34.26±10.36 | 30.50±7.42 | 30.30±10.16 | 0.301 | 0.159 | 0.953 | 0.247 | |
| Gender (female/male) | 14/12 | 6/8 | 11/12 | 0.665 | - | - | - | |
| Education level (yr) | 13.35±3.22 | 14.57±3.46 | 13.43±2.74 | 0.457 | 0.921 | 0.286 | 0.240 | |
| HAMD (before) | 25.12±4.38 | 25.64±3.88 | 6.95±5.30 | <0.001* | <0.001* | <0.001* | 0.733 | |
| HAMD (after) | 9.04±2.63 | 17.86±3.96 | - | - | - | - | <0.001* | |
| Medications | ||||||||
| Trail 1 (TCA/SNRI/SSRI) | 1-8-17 | 1/4/9 | - | - | - | - | - | |
| Trail 2 (TCA/SNRI/SSRI) | - | 1/10/3 | - | - | - | - | - | |
| Vegetables | ||||||||
| Corrected word | 7.50±2.18 | 7.93±2.49 | 10.22±2.65 | 0.001* | <0.001* | 0.007* | 0.596 | |
| Accuracy | 90.95±11.39 | 88.91±16.49 | 95.40±9.12 | 0.234 | 0.199 | 0.115 | 0.610 | |
| Family applications | ||||||||
| Corrected word | 8.62±1.68 | 9.43±2.06 | 11.48±2.27 | 0.001* | <0.001* | 0.004* | 0.224 | |
| Accuracy | 95.85±7.45 | 94.16±6.78 | 96.36±5.18 | 0.602 | 0.788 | 0.325 | 0.437 | |
| Four-foot animals | ||||||||
| Corrected word | 8.12±1.61 | 8.14±1.51 | 10.35±2.17 | <0.001* | <0.001* | 0.001* | 0.964 | |
| Accuracy | 91.99±9.71 | 92.16±9.49 | 95.41±6.47 | 0.335 | 0.170 | 0.270 | 0.953 | |
| Fruits | ||||||||
| Corrected word | 7.34±1.62 | 8.28±2.02 | 9.69±1.74 | <0.001* | <0.001* | 0.021* | 0.112 | |
| Accuracy | 94.43±9.31 | 95.09±12.72 | 95.79±6.91 | 0.881 | 0.616 | 0.837 | 0.834 | |
REFERENCES
1. Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D project results: a comprehensive review of findings. Curr Psychiatry Rep 2007;9:449-459.



2. Greden JF. The burden of disease for treatment-resistant depression. J Clin Psychiatry 2001;62 Suppl 16:26-31.

3. Kubitz N, Mehra M, Potluri RC, Garg N, Cossrow N. Characterization of treatment resistant depression episodes in a cohort of patients from a US commercial claims database. PLoS One 2013;8:e76882.



4. Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage 2010;50:347-356.


5. Trivedi MH, Hollander E, Nutt D, Blier P. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J Clin Psychiatry 2008;69:246-258.


6. Duhameau B, Ferré JC, Jannin P, Gauvrit JY, Vérin M, Millet B, et al. Chronic and treatment-resistant depression: a study using arterial spin labeling perfusion MRI at 3 tesla. Psychiatry Res 2010;182:111-116.


7. Yamamura T, Okamoto Y, Okada G, Takaishi Y, Takamura M, Mantani A, et al. Association of thalamic hyperactivity with treatment-resistant depression and poor response in early treatment for major depression: a resting-state fMRI study using fractional amplitude of low-frequency fluctuations. Transl Psychiatry 2016;6:e754.




8. Li CT, Su TP, Wang SJ, Tu PC, Hsieh JC. Prefrontal glucose metabolism in medication-resistant major depression. Br J Psychiatry 2015;206:316-323.


9. Pallanti S, Di Rollo A, Antonini S, Cauli G, Hollander E, Quercioli L. Low-frequency rTMS over right dorsolateral prefrontal cortex in the treatment of resistant depression: cognitive improvement is independent from clinical response, resting motor threshold is related to clinical response. Neuropsychobiology 2012;65:227-235.



10. Richieri R, Jouvenoz D, Verger A, Fiat P, Boyer L, Lançon C, et al. Changes in dorsolateral prefrontal connectivity after rTMS in treatment-resistant depression: a brain perfusion SPECT study. Eur J Nucl Med Mol Imaging 2017;44:1051-1055.



11. Furtado CP, Hoy KE, Maller JJ, Savage G, Daskalakis ZJ, Fitzgerald PB. Cognitive and volumetric predictors of response to repetitive transcranial magnetic stimulation (rTMS) - a prospective follow-up study. Psychiatry Res 2012;202:12-19.


12. Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev 2009;33:699-771.



13. Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res 2009;174:81-88.



14. Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage 2004;23 Suppl 1:S275-S288.


15. Pu S, Matsumura H, Yamada T, Ikezawa S, Mitani H, Adachi A, et al. Reduced frontopolar activation during verbal fluency task associated with poor social functioning in late-onset major depression: multichannel near-infrared spectroscopy study. Psychiatry Clin Neurosci 2010;62:728-737.

16. Akiyama T, Koeda M, Okubo Y, Kimura M. Hypofunction of left dorsolateral prefrontal cortex in depression during verbal fluency task: a multi-channel near-infrared spectroscopy study. J Affect Disord 2018;231:83-90.


17. Noda T, Yoshida S, Matsuda T, Okamoto N, Sakamoto K, Koseki S, et al. Frontal and right temporal activations correlate negatively with depression severity during verbal fluency task: a multi-channel near-infrared spectroscopy study. J Psychiatr Res 2012;46:905-912.


18. Liu X, Sun G, Zhang X, Xu B, Shen C, Shi L, et al. Relationship between the prefrontal function and the severity of the emotional symptoms during a verbal fluency task in patients with major depressive disorder: a multi-channel NIRS study. Prog Neuropsychopharmacol Biol Psychiatry 2014;54:114-121.


19. Kiriyama T, Tanemura R, Nakamura Y, Takemoto C, Hashimoto M, Utsumi H. Reduced temporal activation during a verbal fluency task is associated with poor motor speed in patients with major depressive disorder. Psychiatry Investig 2020;17:804-813.




20. Tsujii N, Mikawa W, Adachi T, Sakanaka S, Shirakawa O. Right prefrontal function and coping strategies in patients with remitted major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2021;108:110085


21. C Schudlo L, Chau T. Towards a ternary NIRS-BCI: single-trial classification of verbal fluency task, stroop task and unconstrained rest. J Neural Eng 2015;12:066008



22. Kahlaoui K, Di Sante G, Barbeau J, Maheux M, Lesage F, Ska B, et al. Contribution of NIRS to the study of prefrontal cortex for verbal fluency in aging. Brain Lang 2012;121:164-173.


23. Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry 2007;52:46-54.



25. Sun JJ, Liu XM, Shen CY, Zhang XQ, Sun GX, Feng K, et al. Reduced prefrontal activation during verbal fluency task in chronic insomnia disorder: a multichannel near-infrared spectroscopy study. Neuropsychiatr Dis Treat 2017;13:1723-1731.




26. Sun JJ, Liu XM, Shen CY, Feng K, Liu PZ. Abnormal prefrontal brain activation during a verbal fluency task in bipolar disorder patients with psychotic symptoms using multichannel NIRS. Neuropsychiatr Dis Treat 2018;14:3081-3090.




27. Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10-20 system oriented for transcranial functional brain mapping. Neuroimage 2004;21:99-111.


28. Hori H, Ozeki Y, Terada S, Kunugi H. Functional near-infrared spectroscopy reveals altered hemispheric laterality in relation to schizotypy during verbal fluency task. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:1944-1951.


29. Kono T, Matsuo K, Tsunashima K, Kasai K, Takizawa R, Rogers MA, et al. Multiple-time replicability of near-infrared spectroscopy recording during prefrontal activation task in healthy men. Neurosci Res 2007;57:504-512.


30. Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. Neuroimage 2009;44:428-447.


31. Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage 2006;29:368-382.



32. Fisk JE, Sharp CA. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J Clin Exp Neuropsychol 2004;26:874-890.


33. Bozikas VP, Kosmidis MH, Karavatos A. Disproportionate impairment in semantic verbal fluency in schizophrenia: differential deficit in clustering. Schizophr Res 2005;74:51-59.


34. Rajtar-Zembaty A, Sałakowski A, Rajtar-Zembaty J, Starowicz-Filip A. Executive dysfunction in late-life depression. Psychiatr Pol 2017;51:705-718.



35. Ahern E, Semkovska M. Cognitive functioning in the first-episode of major depressive disorder: a systematic review and meta-analysis. Neuropsychology 2016;31:52-72.


36. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a metaanalysis and review. Psychol Bull 2013;139:81-132.



37. Porter RJ, Gallagher P, Thompson JM, Young AH. Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry 2018;182:214-220.

38. Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: evidence of modest impairment. Biol Psychiatry 2001;50:35-43.



39. Akiyama T, Koeda M, Okubo Y, Kimura M. Hypofunction of left dorsolateral prefrontal cortex in depression during verbal fluency task: a multi-channel near-infrared spectroscopy study. J Affect Disor 2018;231:83-90.

40. Noda T, Yoshida S, Matsuda T, Okamoto N, Sakamoto K, Koseki S, et al. Frontal and right temporal activations correlate negatively with depression severity during verbal fluency task: a multi-channel near-infrared spectroscopy study. J Psychiatr Res 2012;46:905-912.


41. Kinou M, Takizawa R, Marumo K, Kawasaki S, Kawakubo Y, Fukuda M, et al. Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophr Res 2013;150:459-467.


42. Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry 2013;70:1181-1189.



43. Matsuo K, Kato N, Kato T. Decreased cerebral haemodynamic response to cognitive and physiological tasks in mood disorders as shown by near-infrared spectroscopy. Psychol Med 2002;32:1029-1037.


44. Matsubara T, Matsuo K, Nakashima M, Nakano M, Harada K, Watanuki T, et al. Prefrontal activation in response to emotional words in patients with bipolar disorder and major depressive disorder. Neuroimage 2014;85 Pt 1:489-497.


45. Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007;62:429-437.



46. Masuda K, Nakanishi M, Okamoto K, Kawashima C, Oshita H, Inoue A, et al. Different functioning of prefrontal cortex predicts treatment response after a selective serotonin reuptake inhibitor treatment in patients with major depression. J Affect Disord 2017;214:44-52.


47. Samson AC, Meisenzahl E, Scheuerecker J, Rose E, Schoepf V, Wiesmann M, et al. Brain activation predicts treatment improvement in patients with major depressive disorder. J Psychiatr Res 2011;45:1214-1222.


48. Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng LL, et al. A treatmentresistant default mode subnetwork in major depression. Biol Psychiatry 2013;74:48-54.









