 |
 |
- Search
| Psychiatry Investig > Volume 20(5); 2023 > Article |
|
Abstract
Objective
Anesthesia-induced cognitive impairments are common for elder patients after surgery. Oxidative stress is the predominant factor contributing to the impairments. This study was to assess the therapeutic potential of an anti-oxidative naturally occurring flavonoid, chrysin, in attenuating sevoflurane-induced cognitive impairments in rat models.
Methods
Rat models of cognitive impairments were constructed by exposing aged rats (18 months old) to sevoflurane for 2 h. Chrysin was administered via oral gavage at the dose of 25, 50, and 100 mg/kg/day for seven days. The elevated plus maze test was used to assess anxiety and explorative behaviors. Spatial memory tests were performed using novel object recognition test, object location memory task, and water maze experiments. Oxidative stress was evaluated by measuring levels of malondialdehyde, nicotinamide adenine dinucleotide phosphate, 4-hydroxynonenal, and glutathione using colorimetric assays. Quantitative real-time polymerase chain reaction and Western blot were used to analyze how chrysin affects nuclear factor E2-related factor (Nrf) signaling.
Results
While sevoflurane anesthesia led to significant decline in cognitive performance in object recognition test, object location memory task, and water maze test, chrysin exerted significant effects in alleviating the impairments. Oxidative stress was also reduced in the hippocampus tissue of rats after chrysin intake. Nrf signaling was activated by chrysin treatment in sevoflurane-induced cognitive impairment models.
Postoperative cognitive dysfunctions (POCD) are cognitive disorders following anesthesia and surgery, which are common in older patients who already are subject to gradual loss of cognitive performance [1]. POCD have the characteristics including exacerbation in cognitive functions, progressive hypomnesia, and personality alteration. Despite that anesthetics such as sevoflurane and propofol have been widely used in clinics as inhalation anesthetics for maintenance of general anesthesia, it has been revealed lately that anesthesia is an important factor contributing to behavioral outcomes such as POCD in patients [2,3]. With older patients, who are more susceptible to anesthesia-related cognitive impairment, representing an increasing proportion of patients undergoing surgical procedures, it is critical to develop interventions that effectively protect from or mitigate detrimental effects of anesthesia.
Oxidative stress is deemed a significant factor in the pathophysiology of a variety of cognitive disorders [4]. Reactive oxygen species (ROS) are thought of as an important player in the cognitive damage as excess ROS, which induce damages of cellular macromolecules, can harm cellular proteins, DNA, and lipids, thus disrupting normal physiological functions [5]. Strategies that alleviate oxidative stress are considered promising in treating cognitive decline and impairments in various clinical settings, including POCD [6].
Pharmacologic interventions are the mainstay of antioxidative stress treatments [7]. One class of drugs are flavonoids, which are plant-derived polyphenolic compounds [8]. Thus far, several flavonoids have been reported efficacious in cognitive disorders. For example, hispidulin (4',5,7-trihydroxy-6-methoxyflavone) was shown to have a protective role against sevoflurane-induced memory loss in aged rats [9]. Nobiletin (5,6,7,8,3',4'-hexamethoxyflavone) was also reported to anesthesia-induced neurocognitive decline [10]. Chrysin (5,7-dihydroxy flavones) is a naturally-occurring flavone found in flowers, honey, mushrooms, and propolis [11]. The anxiolytic [12], antioxidant [13], antidiabetogenic [14], anti-cancer [15], and antiestrogenic [16] properties of chrysin have been reported. For example, antiaging effect of chrysin has been investigated in d-galactose-induced aging model in rats [13]. The efficacies of chrysin to protect against cognitive, behavioral, and neurochemical changes have been reported in a doxorubicin-induced model of Parkinson’s disease [17]. The neuroprotective role of chrysin can also be manifested by that chrysin mitigate brain injury-induced cognitive decline [18]. However, it is unknown whether chrysin can alleviate cognitive impairment caused by anesthesia.
Herein, the aim of the present work was to evaluate the effect of chrysin intervention against cognitive impairment in cognitively impaired aged rats induced by sevoflurane, as a model anesthetic agent. We focused on evaluating cognitive performance of rats with cognitive impairment and the efficacies of chrysin in alleviating the impairments. The present study also evaluates the reduction of oxidative stress exerted by chrysin, and explore how nuclear factor E2-related factor 2 (Nrf2) signaling, a putative molecular pathway associated with oxidative stress in the brain [19], was affected by chrysin treatment. The results of the study could help elucidate the potential of chrysin as a new biocompatible pharmacologic agent for alleviating POCD.
The male aged standard deviation (SD) rats (18 months of age) were purchased from GemPharmatech (Nanjing, China). Male SD rats of 18-month old were treated with inhalation of 3% sevoflurane (Sigma-Aldrich, St. Louis, MO, USA) for 2 h [20] to induce cognitive impairment. All animal experiments were performed in compliance to the regulations of Quanzhou First Hospital Affiliated to Fujian Medical University. Chrysin (Sigma-Aldrich) dissolved in phosphate-buffered saline containing 3% (v/v) NaOH (0.01 M) was administered to the rats using the doses of 25, 50, and 100 mg/kg/day by oral gavage [21]. Cognitive and biochemical tests were performed on rats seven days after treatments.
The rats were fully habituated to the testing apparatus before experiments. The animals were firstly trained after being individually placed in the center of the apparatus, in which two identical objects were placed, one on each side. After a 4-min free exploration period, and staying in cages for another 2 h or 24 h, the rats were placed in the same apparatus and exposed to a novel object in addition to a familiar object. The recognition index was calculated as the proportion of time spent with the novel object. Object location memory (OLM) task differed from the novel object recognition (NOR) in that spatial markers were made on the wall during OLM test to help the rats make spatial judgments.
The elevated plus maze test was used to assess explorative behaviors and anxiety. Briefly, animals were allowed to explore the open and closed arms of a plus-shaped maze for 5 min after being placed at the junction and facing close arms. The time ratio (RT) was defined as RT=TO/(TO+TC), where TO and TC represent the time spent in open arms and close arms, respectively.
The water maze experiment was performed using previously described protocols [22]. Briefly, rats were placed at four fixed entry points of a water pool (110 cm diameter) filled with opaque water and divided into four virtual quadrants: left, right, opposite, and target. The rats underwent 3 days of cued training, five days of acquisition training and a final probe trial. During the final probe trial, the trajectory and the time rats took to find a center circular platform (10 cm) were recorded and 1 min in total was allowed to find the center platform. Water temperature was maintained at 20°C±2°C. Key parameters evaluated included escape latencies, the average swim speed, the number of platform site crossings, and the time in the target quadrant. After training and trial, the animals were kept warm using a heat lamp to prevent hypothermia until they dried. A tracking software (Stoelting Co., Wood Dale, IL, USA) was used to capture the trajectory of rats.
Following behavioral tests, rats were euthanized by intraperitoneal injecting 150 mg/kg pentobarbital sodium and hippocampal tissues were rapidly collected and homogenized using 50 mM Tris-HCl (pH 7.4). After centrifuging the homogenate at 2,400g for 15 min at 4°C, supernatant was collected for assays. Oxidative stress markers, including malondialdehyde (MDA), nicotinamide adenine dinucleotide phosphate (NADPH), 4-hydroxynonenal (HNE), and glutathione (GSH) were analyzed as previously described [17].
Hippocampus tissues were also analyzed using real-time polymerase chain reaction and Western blot using procedures as previously described [23,24]. Primer sequences used in our study included: Nrf2: forward, GCGCAGACATTCCC GTTTGT, reverse, GCTCTCGATGTGACCGGGAA; heme oxygenase-1 (HO-1): forward, TGGTGGCGACAGTTGCT GTA, reverse, AAGGCCTTCCACCGGACAAA; NAD(P) H:quinone oxidoreductase (NQO)-1: forward, CTACGATC CGCCCCCAACTT, reverse, CCTCTCTGCGTGGGC CAATA; glyceraldehyde-3-phosphate dehydrogenase (GAPDH): forward, GCTCCCTCTCTTTCTTTGCAGC, reverse, AGTTGTCATGGATGACCTTGGC.
The values are presented as mean±SD. We performed normal distribution analysis for all the data in the behavioral experiments using the Anderson-Darling test, D’Agostino & Pearson test, Shapiro-Wilk test, and Kolmogorov-Smirnov test, and the results showed that the data were satisfying the normal distribution. Because the SD values of each group were different, we used the the Brown-Forsythe ANOVA test was used to analyze differences between groups using Prism GraphPad (GraphPad Software, San Diego, CA, USA), with post-hoc testing conducted using the Dunn’s multiple comparisons test or Tukey’s multiple comparisons test. p<0.05 were considered statistically significant.
All animal experiments were performed in compliance to the regulations of Quanzhou First Hospital Affiliated to Fujian Medical University (approval number: QZYY-2019-k38). This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (8th edition, NIH).
We constructed rat models of cognitive function impairment using sevoflurane induction for 2 h, which were treated with varying doses of chrysin for seven days. After seven days, the anxiety behaviors of rats were tested using the elevated plus maze (Figure 1), where the time spent in the center (Figure 1A), open versus closed arms (RT, Figure 1B), distal open arms (Figure 1C), and total distance traveled (Figure 1D) were recorded. It was seen that sevoflurane anesthesia led to severe anxiety behavior in rats, elevated by increased time in center, and decreased TC, distal open arms, and total distance, but chrysin significantly improved anxiety in rats with a dose-dependent manner. No side effects of chrysin, gauged by body weight, aberrant behavior, etc., compared to the control group were observed.
The spatial memory functions of the rats were also analyzed using the OLM task (Figure 2A and B) and NOR assays (Figure 2C and D). Our results indicated that sevoflurane anesthesia led to steep decline in the recognition indexes in NOR and OLM assays. But following continuous chrysin administration for seven days, significantly restored spatial memory functions could be seen and the restoration effects of chrysin were dose-dependent.
Water maze experiment was also further used to assess the memory function and spatial learning of rats. It can be seen that sevoflurane anesthesia resulted in increased escape latency (Figure 3A), decreased time in the target quadrant (Figure 3C), and platform site crossings (Figure 3D), while average swim speed remained unchanged (Figure 3B). After treating with chrysin, significantly shortened escape latency, increased platform crossing, and time in the target quadrant, could be observed. Sevoflurane treatment also did not alter average swim speed. These data served as another supporting evidence that chrysin attenuates sevoflurane-induced spatial learning and memory impairments.
After treatment with chrysin for seven days, we harvested the hippocampus tissues of the rats and performed analysis of oxidative stress using markers including MDA, NADPH, HNE, and GSH levels (Figure 4). Our data showed that the oxidative stress was heightened after sevoflurane treatment evidenced by increased MDA (Figure 4A), NADPH (Figure 4B), and HNE levels (Figure 4C), as well as lowered GSH levels (Figure 4D). Meanwhile, chrysin was effective in attenuating oxidative stress in the hippocampal tissue of aged rats.
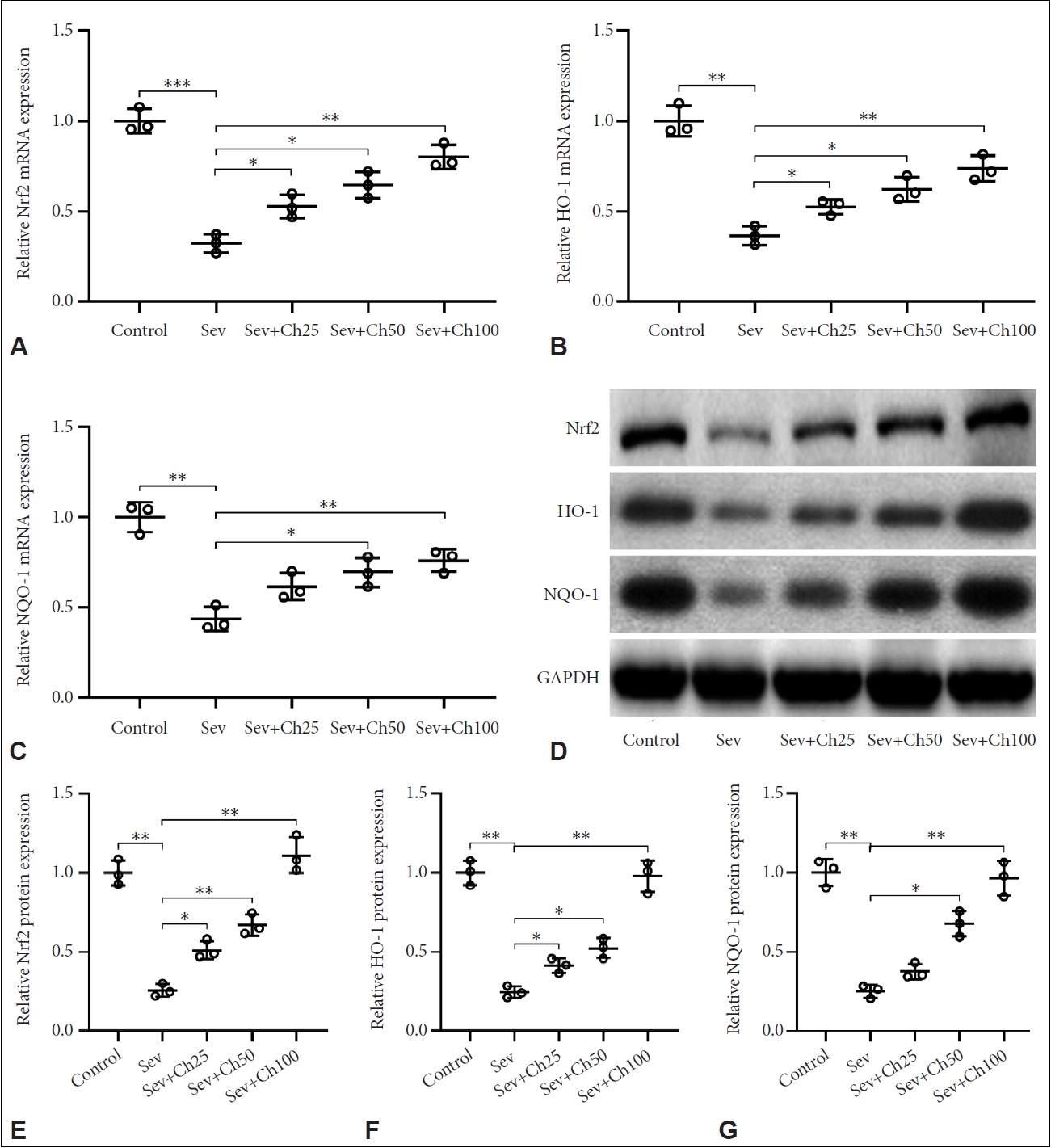
Given the ability of chrysin in regulating oxidative stress in aged rats undergone sevoflurane anesthesia, we next investigated how chrysin affects Nrf2 signaling. Here we showed that downregulation of mRNA levels of Nrf2 (Figure 5A), HO-1 (Figure 5B), and NQO-1 (Figure 5C), which are key molecules in the Nrf signaling pathway, were prominent after sevoflurane treatment, suggesting suppressed Nrf2 signaling. Chrysin treatment, however, restored the levels of Nrf2, HO-1, and NQO-1. Western blot (Figure 5D-G) was further used to confirmed the restoration of Nrf2, HO-1, and NQO-1 on the protein level. Together, these findings suggested that chrysin activates Nrf2 signaling, which alleviates oxidative stress.
With surgery and anesthesia being inevitable medical procedures in certain elderly patients, POCD constitutes a significant risk of cognitive impairment, demanding effective treatment strategies to fill the gap in post-surgery clinical management. Given the significant role of oxidative stress in the pathophysiological process of POCD, in the present study, we focused on an antioxidant, chrysin, to test its efficacies in alleviating sevoflurane-induced cognitive impairment in rat models. Our data suggested that chrysin is a potent naturally-occurring flavonoid that markedly improved cognitive performance of rats after exposing to sevoflurane anesthesia. The results echoed previous findings that showed the potential of chrysin for treating a number of cerebral/cognitive diseases [13,23,25]. For example, in animal models of dementia and neurodegeneration induced by decreased cerebral blood flow, chrysin has shown efficacies presumably due to its antioxidant properties [25]. Further, it was found that chrysin adopts a protective role against oxidative stress-induced tissue damage in d-galactose-induced aging rat models [13]. Besides, chrysin was shown to improve cognitive, behavioral, and neurochemical parameters in a mouse model of Parkinson’s disease [17]. Using elevated plus maze test, NOR test, and water maze test, our study performed a comprehensive behavioral and cognitive evaluation of the efficacies of chrysin in alleviating anesthesia-induced cognitive impairment in rats, for the first time showing that chrysin of potential clinical utility in POCD. It is worth noting that aged rages were chosen to make our study consistent with the fact that anesthesia related cognitive impairment is a common occurrence in elderly surgical patients. About a quarter of elderly patients greater than 60 years old have been reported to have POCD a week after surgery, which persists for 3 months or more in 10 % of these affected patients [1].
We attributed the efficacies of chrysin in alleviating cognitive impairment to its role as an antioxidant. A number of previous studies have investigated the role of chrysin as a free radical scavenger and modulator of brain-derived neurotrophic factor production, which likely contributed to its effect in preventing age-associated memory loss and progression of Parkinson’s disease [17,26]. By evaluating oxidative stress in hippocampus, the cognitive central, at the tissue level, our study provided solid proof that chrysin indeed lowered oxidative stress. Further, Nrf signaling, which is a putative pathway associated with oxidative stress, has also shown to be activated by chrysin. In previous studies, a number of flavonoids have shown to activate Nrf signaling and our study expands our knowledge on the significance of chrysin as a Nrf activator, which could have implications in other neurological diseases, where Nrf is suppressed. It should also be noted that TLR4-NF-kB(p65)-NLRP3 pathways, among other pathways, are also regulated by chrysin [27], and it is likely that the potency of chrysin in alleviating cognitive impairment stems from multiple mechanism.
Our study is limited in several aspects. First, sevoflurane is one of the anesthetic agents used clinically and it is worth testing the efficacy of chrysin in cognitive impairment models induced by other anesthetic agent. Moreover, the protective role of chrysin in POCD remains to be tested as the intake of chrysin before injury have been shown to prevent cognitive impairments due to cerebral ischemia [18,22]. The optimal dose of chrysin and the routes of chrysin intake should also be optimized as systemic administration, such as intravenous injection, could likely lead to higher bioavailability. Novel delivery methods, such as chrysin transfersomal and composite vesicles to facilitate chrysin’s nose-to-brain delivery [27], are also worth exploring to maximize benefits for patients with POCD. Notably, we have now found that chrysin activated Nrf2 signal in hippocampus of aged rats after sevoflurane anesthesia and was able to reduce oxidative stress, but it remains to be clarified whether other pathways contribute to the effect. Therefore, in future studies, we will explore other possible mechanisms which chrysin utilizes to improve cognitive impairment in anesthesia-induced aged rats. For example, the roles of inflammatory response, autophagy, and modulation of the immune microenvironment are worth exploring. Meanwhile, Nrf2 inhibitors could be used on chrysin-treated animals to observe whether chrysin still has a protective effect after Nrf2 inhibition, thus verifying the key mechanisms of Nrf2 activation by chrysin.
In conclusion, by evaluating the efficacy of chrysin against sevoflurane-induced cognitive impairment in aged rats, we show that chrysin improved performance of impaired rats in elevated plus maze test, NOR test, and water maze test. On tissue level, chrysin reduces oxidative stress through the activation of Nrf signaling. Our data support further development of chrysin as a pharmacologic intervention in clinical management of POCD.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Shunyuan Li. Data curation: Caiping Chen, Jingyang Zeng, Bo Luo. Investigation: Caiping Chen, Jingyang Zeng, Bo Luo. Project administration: Shunyuan Li. Resources: Shunyuan Li. Supervision: Shunyuan Li. Validation: Caiping Chen. Writing—original draft: all authors. Writing—review & editing: Shunyuan Li.
Funding Statement
None
Figure 1.
Effects of chrysin on sevoflurane-induced anxiety in aged rats. (A) Chrysin decreased the time spent in center. (B) Chrysin increased the time spent on the open arms and the time spent in the distal parts of the open arms (C) as well as total distance traveled (D). N=10 rats for each group. Brown-Forsythe ANOVA followed Dunn’s multiple comparisons test. *p<0.05; **p<0.01; ***p<0.001. Sev, sevoflurane; Ch25, chrysin 25 mg/kg/day; Ch50, chrysin 50 mg/kg/day; Ch100, chrysin 100 mg/kg/day.

Figure 2.
Effects of chrysin on sevoflurane anesthesia-induced cognitive impairments of aged rats, determined by novel object recognition (NOR) assay (A and B) and object location memory (OLM) task (C and D). The recognition index was calculated as the proportion of time with the target or novel object out of the total time. N=10 rats for each group. Brown-Forsythe ANOVA followed Dunn’s multiple comparisons test. *p<0.05; **p<0.01; ***p<0.001. Sev, sevoflurane; Ch25, chrysin 25 mg/kg/day; Ch50, chrysin 50 mg/kg/day; Ch100, chrysin 100 mg/kg/day.
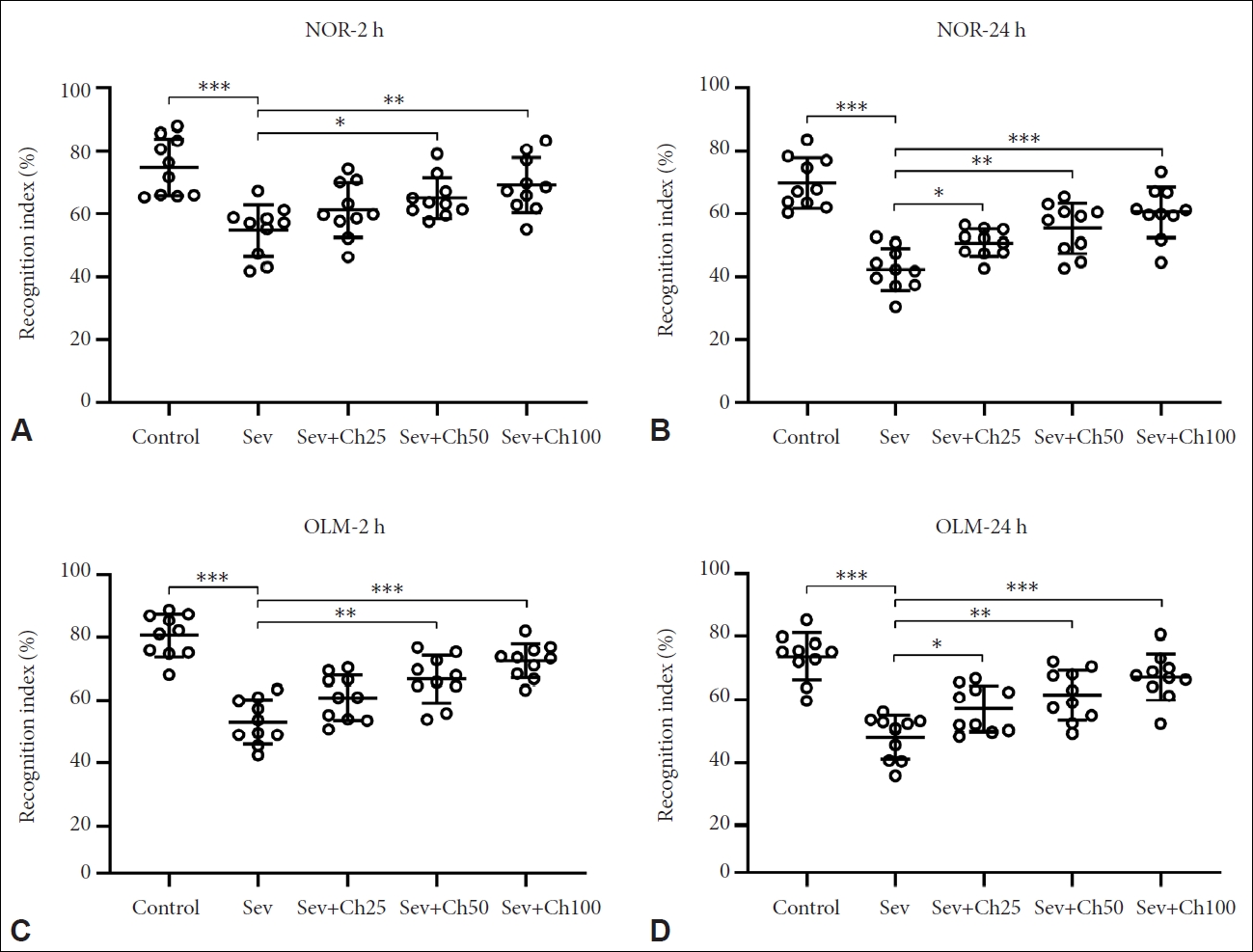
Figure 3.
Effects of chrysin on sevoflurane anesthesia-induced spatial learning and memory impairments of aged rats by Morris Water Maze test. The escape latencies (A) and the average swim speed (B) were recorded based on three training sessions. The time in the target quadrant (C) and the number of platform site crossings during 60 s of test (D) were recorded. N=10 rats for each group. Brown-Forsythe ANOVA followed Dunn’s multiple comparisons test. *p<0.05; **p<0.01; ***p<0.001. Sev, sevoflurane; Ch25, chrysin 25 mg/kg/day; Ch50, chrysin 50 mg/kg/day; Ch100, chrysin 100 mg/kg/day.
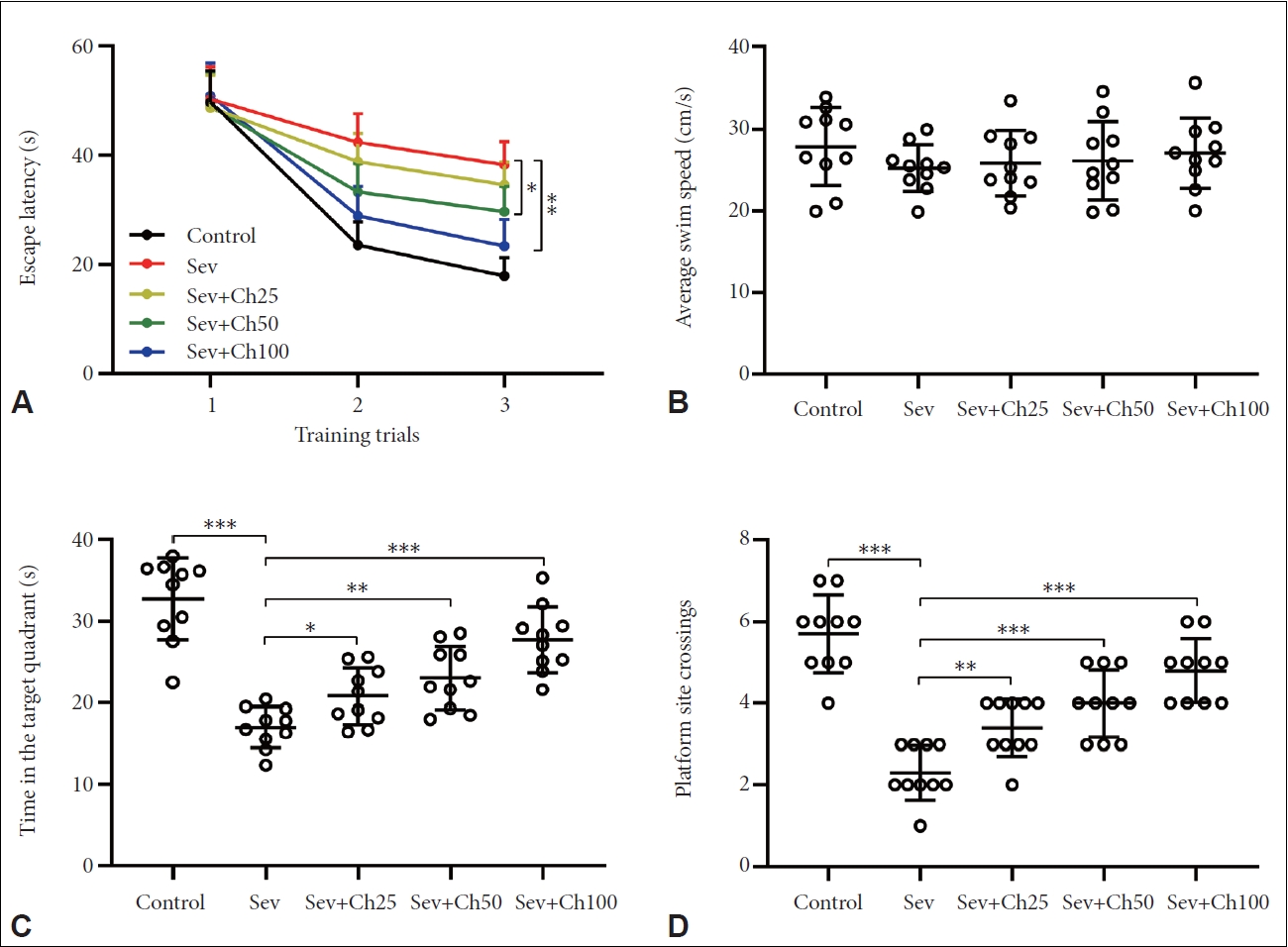
Figure 4.
Effects of chrysin on sevoflurane anesthesia-induced oxidative stress in hippocampus of aged rats. Levels of MDA (A), NADPH oxidase activity (B), HNE levels (C) and GSH activity (D) in hippocampus among different groups were measured. N=10 rats for each group. Data were presented with mean±standard deviation. Brown-Forsythe ANOVA followed Dunn’s multiple comparisons test. *p<0.05; **p<0.01; ***p<0.001. MDA, malondialdehyde; NADPH, nicotinamide adenine dinucleotide phosphate; HNE, hydroxynonenal; GSH, glutathione; Sev, sevoflurane; Ch25, chrysin 25 mg/kg/day; Ch50, chrysin 50 mg/kg/day; Ch100, chrysin 100 mg/kg/day.

Figure 5.
Nrf2 signal in hippocampus of aged rats after sevoflurane anesthesia upon chrysin treatment. The levels of Nrf2 (A), HO-1 (B) and NQO-1 (C) mRNAs in hippocampus of aged rats after sevoflurane anesthesia were tested by RT-qPCR. (D) The protein levels of Nrf2, HO-1 and NQO-1 in hippocampus of aged rats after sevoflurane anesthesia were measured using western blot. GAPDH was used as a loading control and the expressions were normalized to control (E-G). N=3 repeats for each group (10 tissue homogenates were mixed for each group). Brown-Forsythe ANOVA followed Dunn’s multiple comparisons test.*p<0.05; **p<0.01; ***p<0.001. Nrf2, nuclear factor E2-related factor 2; HO-1, heme oxygenase-1; NQO-1, NAD(P)H:quinone oxidoreductase-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Sev, sevoflurane; Ch25, chrysin 25 mg/kg/day; Ch50, chrysin 50 mg/kg/day; Ch100, chrysin 100 mg/kg/day.
REFERENCES
1. Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction - current preventive strategies. Clin Interv Aging 2018;13:2267-2273.


2. Wang CM, Chen WC, Zhang Y, Lin S, He HF. Update on the mechanism and treatment of sevoflurane-induced postoperative cognitive dysfunction. Front Aging Neurosci 2021;13:702231



3. Konishi Y, Evered LA, Scott DA, Silbert BS. Postoperative cognitive dysfunction after sevoflurane or propofol general anaesthesia in combination with spinal anaesthesia for hip arthroplasty. Anaesth Intensive Care 2018;46:596-600.



4. Luca M, Luca A. Oxidative stress-related endothelial damage in vascular depression and vascular cognitive impairment: beneficial effects of aerobic physical exercise. Oxid Med Cell Longev 2019;2019:8067045




5. Madreiter-Sokolowski CT, Thomas C, Ristow M. Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol 2020;36:101678



6. Wei P, Yang F, Zheng Q, Tang W, Li J. The potential role of the NLRP3 inflammasome activation as a link between mitochondria ROS generation and neuroinflammation in postoperative cognitive dysfunction. Front Cell Neurosci 2019;13:73



7. Hall ED, Wang JA, Miller DM, Cebak JE, Hill RL. Newer pharmacological approaches for antioxidant neuroprotection in traumatic brain injury. Neuropharmacology 2019;145(Pt B):247-258.


8. Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem 2022;383:132531


9. Huang L, Huang K, Ning H. Hispidulin prevents sevoflurane— induced memory dysfunction in aged rats. Biomed Pharmacother 2018;97:412-422.


10. Sun Z, Yang N, Jia X, Song Y, Han D, Wang X, et al. Nobiletin attenuates anesthesia/surgery-induced neurocognitive decline by preserving the expression of clock genes in mice. Front Neurosci 2022;16:938874



11. Ileriturk M, Benzer F, Aksu EH, Yildirim S, Kandemir FM, Dogan T, et al. Chrysin protects against testicular toxicity caused by lead acetate in rats with its antioxidant, anti-inflammatory, and antiapoptotic properties. J Food Biochem 2021;45:e13593



12. Rodríguez-Landa JF, German-Ponciano LJ, Puga-Olguín A, OlmosVázquez OJ. Pharmacological, neurochemical, and behavioral mechanisms underlying the anxiolytic-and antidepressant-like effects of flavonoid chrysin. Molecules 2022;27:3551



13. Anand KV, Mohamed Jaabir MS, Thomas PA, Geraldine P. Protective role of chrysin against oxidative stress in d-galactose-induced aging in an experimental rat model. Geriatr Gerontol Int 2012;12:741-750.

14. Dewi RM, Megawati M, Antika LD. Antidiabetic properties of dietary chrysin: a cellular mechanism review. Mini Rev Med Chem 2022;22:1450-1457.



15. Talebi M, Talebi M, Farkhondeh T, Simal-Gandara J, Kopustinskiene DM, Bernatoniene J, et al. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin. Cancer Cell Int 2021;21:214




16. van Meeuwen JA, Korthagen N, de Jong PC, Piersma AH, van den Berg M. (Anti)estrogenic effects of phytochemicals on human primary mammary fibroblasts, MCF-7 cells and their co-culture. Toxicol Appl Pharmacol 2007;221:372-383.


17. Del Fabbro L, Rossito Goes A, Jesse CR, de Gomes MG, Cattelan Souza L, Lobo Ladd FV, et al. Chrysin protects against behavioral, cognitive and neurochemical alterations in a 6-hydroxydopamine model of Parkinson’s disease. Neurosci Lett 2019;706:158-163.


18. El Khashab IH, Abdelsalam RM, Elbrairy AI, Attia AS. Chrysin attenuates global cerebral ischemic reperfusion injury via suppression of oxidative stress, inflammation and apoptosis. Biomed Pharmacother 2019;112:108619


19. Khan A, Ikram M, Muhammad T, Park J, Kim MO. Caffeine modulates cadmium-induced oxidative stress, neuroinflammation, and cognitive impairments by regulating Nrf-2/HO-1 in vivo and in vitro. J Clin Med 2019;8:680



20. Liu P, Wang J, Peng S, Zhang D, Zhuang L, Liu C, et al. Suppression of phosphodiesterase IV enzyme by roflumilast ameliorates cognitive dysfunction in aged rats after sevoflurane anaesthesia via PKA-CREB and MEK/ERK pathways. Eur J Neurosci 2022;56:4317-4332.



21. Rashno M, Ghaderi S, Nesari A, Khorsandi L, Farbood Y, Sarkaki A. Chrysin attenuates traumatic brain injury-induced recognition memory decline, and anxiety/depression-like behaviors in rats: insights into underlying mechanisms. Psychopharmacology (Berl) 2020;237:1607-1619.



22. Sarkaki A, Farbood Y, Mansouri SMT, Badavi M, Khorsandi L, Dehcheshmeh MG, et al. Chrysin prevents cognitive and hippocampal long-term potentiation deficits and inflammation in rat with cerebral hypoperfusion and reperfusion injury. Life Sci 2019;226:202-209.


23. Çelik H, Kucukler S, Çomaklı S, Caglayan C, Özdemir S, Yardım A, et al. Neuroprotective effect of chrysin on isoniazid-induced neurotoxicity via suppression of oxidative stress, inflammation and apoptosis in rats. Neurotoxicology 2020;81:197-208.


24. Li T, Zheng LN, Han XH. Fenretinide attenuates lipopolysaccharide (LPS)-induced blood-brain barrier (BBB) and depressive-like behavior in mice by targeting Nrf-2 signaling. Biomed Pharmacother 2020;125:109680


25. He XL, Wang YH, Bi MG, Du GH. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur J Pharmacol 2012;680:41-48.


26. Souza LC, Antunes MS, Filho CB, Del Fabbro L, de Gomes MG, Goes AT, et al. Flavonoid chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol Biochem Behav 2015;134:22-30.


27. Ibrahim SS, Abo Elseoud OG, Mohamedy MH, Amer MM, Mohamed YY, Elmansy SA, et al. Nose-to-brain delivery of chrysin transfersomal and composite vesicles in doxorubicin-induced cognitive impairment in rats: insights on formulation, oxidative stress and TLR4/NF-kB/NLRP3 pathways. Neuropharmacology 2021;197:108738









