A Systematic Review of Pharmacological Treatments for Internet Gaming Disorder
Article information
Abstract
Objective
Internet gaming disorder (IGD) is an increasingly common behavioral addiction, with an estimated global prevalence of 3%. A variety of pharmacological treatments have been used to treat IGD, yet no review to date has synthesized clinical trials evaluating their efficacy. This systematic review therefore synthesized the literature reporting on clinical trials of pharmacological treatments for IGD.
Methods
We reviewed articles from MEDLINE, Embase, PubMed Central, CINAHL, and PsycINFO that were published as of March of 2022. A total of 828 articles were retrieved for review and 12 articles were included, reporting on a total of 724 participants.
Results
Most participants were male (98.6%), and all were currently living in South Korea. The most common drugs used to treat IGD were bupropion, methylphenidate, and a range of selective serotonin reuptake inhibitors. The Young Internet Addiction Scale was the most frequently used to measure gaming-related outcomes. All studies reported reduced symptoms of IGD from pre- to post-treatment. Across all clinical trials, IGD symptom reductions following the administration of pharmacological treatments ranged from 15.4% to 51.4%. A risk of bias assessment indicated that only four studies had a low risk of bias.
Conclusion
Preliminary results suggest that a wide array of pharmacological interventions may be efficacious in the treatment of IGD. Future studies using double-blind randomized controlled trial designs, recruiting larger and more representative samples, and controlling for psychiatric comorbidities are needed to better inform understanding of pharmacological treatments for IGD.
INTRODUCTION
Internet gaming disorder (IGD), now classified as an addictive disorder in the International Classification of Diseases 11th revision [1], is becoming increasingly common, with a recent large-scale meta-analysis indicating global prevalence as high as 3.1% [2]. IGD is characterized by impaired control over gaming, increasing priority given to gaming to the extent that gaming takes precedence over other life interests, and continuation or escalation of gaming despite the occurrence of negative consequences. While IGD falls under a larger umbrella of problematic internet use (PIU), research has identified IGD as unique and distinct from other forms of PIU, such as internet addiction [3,4]; whereas PIU spans a range of online behaviors, including online chatting and social media use, IGD refers specifically to excessive engagement and associated problems with online gaming.
Psychological and pharmacological treatments are the most common treatment options for IGD [5]. Psychological treatments for IGD have predominantly drawn on cognitive-behavioral therapy and have demonstrated good effectiveness in reducing symptoms [6]. Conversely, the types of pharmacological treatments used to treat IGD have varied considerably. Indeed, treatments have included psychostimulants [7], selective serotonin reuptake inhibitors (SSRIs) [8], serotonin and norepinephrine reuptake inhibitors [9], and non-selective inhibitors of dopamine and norepinephrine transporters [10]. To date, empirical research has yet to consolidate the effectiveness of these different drugs, precluding insight as to whether pharmacological treatment is generally effective in reducing symptoms of IGD, and if so, the types that may be most effective.
There is strong evidence to suggest that pharmacological treatments for IGD may be effective, stemming from research demonstrating the functional and structural neurobiological underpinnings of IGD [11]. For example, a recent systematic review found that individuals with IGD display increased activity in brain regions associated with reward, reduced activity in brain regions associated with impulse control, and reduced functional connectivity in brain networks implicated in motivation, reward, and executive function, and cognitive control [12]. This review also identified structural differences among individuals with IGD, with regard to reduced grey-matter volume and white-matter density [12]. Importantly, these neurobiological abnormalities are consistent with those observed in other addictive disorders, such as gambling disorder and substance use disorders—addictive disorders for which pharmacological treatments have demonstrated effectiveness [13-15].
Given increasing understanding of IGD as an addictive disorder and relatively high prevalence rates, an improved understanding of its potential pharmacological treatments is warranted. A previous systematic review [5] examining interventions for IGD identified eight studies that reported on clinical trials of pharmacological interventions for IGD, observing reductions in IGD symptoms following the administration of pharmacological treatments across studies. This review, however, did not focus specifically on pharmacological treatments, thus omitting search terms needed to identify pharmacological treatments and precluding a fulsome identification of relevant studies. As such, we conducted a systematic review of studies examining the effectiveness of pharmacological treatments in reducing symptoms of IGD.
METHODS
Search strategy
The search strategy for this review was developed in consultation with a librarian specializing in systematic reviews. Using MeSH terms, we searched the following databases: MEDLINE, Embase, PubMed Central, CINAHL, and PsycINFO. We included all terms related to IGD and problematic engagement in internet use and or video gaming (e.g., internet gaming disorder, video game addiction). We also included terms related to pharmacological treatment (e.g., pharmacotherapy, medication), as well as terms related to common classes of pharmacological treatments (e.g., selective serotonin reuptake inhibitors, norepinephrine-dopamine reuptake inhibitors) and specific pharmacological treatments (e.g., bupropion, methylphenidate). Terms related to IGD were combined with terms related to pharmacological treatments using Boolean operators. Searches were adapted to each database using appropriate MeSH terms and keywords for each database. We conducted our search on March of 2022 and did not limit results to a specific timeframe. A sample search strategy is provided in Supplementary Materials (in the online-only Data Supplement). The reference lists of the included articles were also screened to identify additional articles reporting on pharmacological treatments for IGD.
Eligibility criteria
We reviewed original articles involving human research participants that reported the use of a pharmacological intervention in the treatment of IGD. Our inclusion criteria were as follows: 1) studies included a sample comprised of people with IGD or gaming-related symptoms if reporting on PIU; 2) studies reported on a pharmacological intervention used in the treatment of IGD; 3) studies reported on at least one outcome measure related to IGD; and 4) studies were available in English, Portuguese, Spanish, or French. Theoretical papers, opinion pieces, commentaries, conference proceedings, case reports, and review papers were excluded, although reference lists of review papers were screened for relevancy.
Screening abstracts
Two authors screened each article independently for relevance based on title and abstract, with discrepancies resolved through consensus. Inter-rater reliability (IRR) between the two authors was near-perfect (Cohen’s kappa=0.85) [16]. Full texts were then retrieved for all included articles and were each independently screened by the two authors, again revealing near-perfect IRR (Cohen’s kappa=0.88). Discrepancies were resolved through consensus and consultation with a third author.
Data extraction
For each included article, the following information were extracted: 1) study design; 2) participant information including sample size, mean age, and sex; 3) instrument used to measure IGD; 4) pharmacological treatments used; 5) duration of treatment; and 6) main results.
Risk of bias
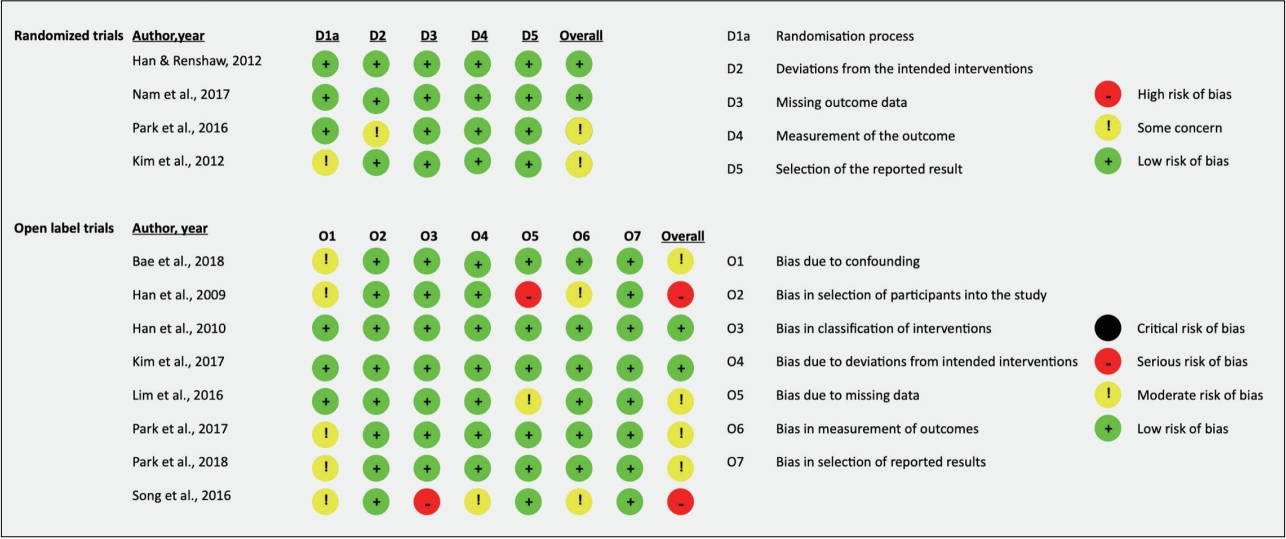
Included manuscripts were assessed by SJ and PP for risk of bias using the Cochrane Collaboration Risk of Bias (ROB-2) Tool [17] for randomized controlled trials and the Cochrane Risk of Bias In Non-Randomized Studies (ROBINS-I) [18] for open label trials. The ROB-2 evaluates the following criteria: random sequence generation, blinding of participants and research personnel, blinding of outcome measures, incomplete outcome data, and selective reporting of results, whereas the ROBINS-I evaluates bias in controlling for confounding variables, selection of participants, classification of interventions, deviations from intended intervention, missing data, measurement of outcomes, and selection of reported results. There was high IRR for each scale (IRR=100%; 90%, respectively). Disagreements were resolved by HSK.
RESULTS
Our search generated 912 articles, of which 828 remained after the removal of duplicates (Figure 1). Of these 828 articles, 19 were retrieved for full-text review. A total of 12 articles met all eligibility criteria and were included in the present review.
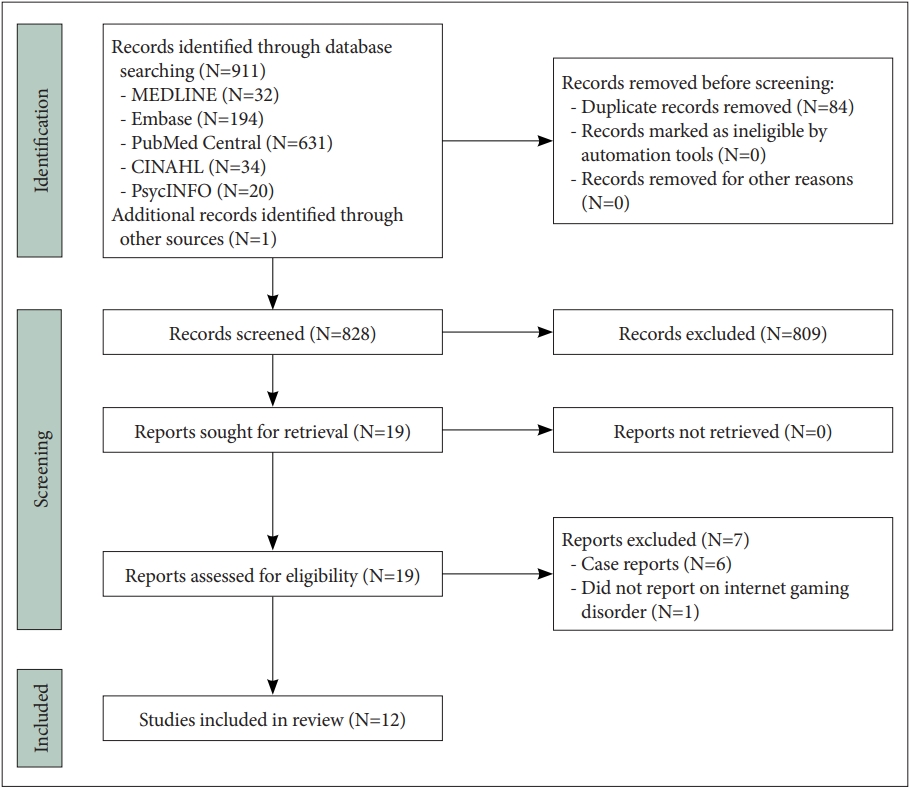
PRISMA flow diagram of the studies included in the present review (N=12). PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Risk of bias
With respect to randomized trials, two of the four included studies were rated as having low risk of bias whereas the remaining two had some concerns, due to the randomization process [19] and deviations from the intended intervention [7]. Comparatively, two of eight open label trials were rated as having low risk of bias, four as having some concerns, and two as having a serious risk of bias. Concerns of bias were primarily due to confounding, with serious concerns in classification of interventions in one study [8] and bias due to missing data in one study [20]. Further details are in Figure 2.
Summary of findings
Table 1 summarizes the main findings of the articles included in the present review. All articles were conducted in South Korea. All studies reported on clinical trials, of which eight were open clinical trials [8,9,19,21-25]; one was an open, uncontrolled clinical trial [20]; one was a prospective, randomized, controlled, single-blind clinical trial [7]; one was a prospective, controlled, double-blind clinical trial [26]; and one was a prospective, randomized, controlled, and double-blind clinical trial [10]. Across all studies, the total sample included 724 participants. Park et al. [7] reported a total of 86 participants in their abstract and 84 participants in their method section. For consistency, we assumed their sample size to be 84 across all our analyses. Of the 694 participants for whom sex was reported, 684 (98.6%) were male and 10 (1.4%) were female. Eight articles reported on samples of adults, three reported on samples of children and/or adolescents, and one reported on both adults and adolescents.
Seven studies focused primarily on the treatment of IGD, totaling 63.6% (n=461) of participants with IGD across all articles. Five of the seven studies included participants with IGD and other psychiatric comorbidities (n=298), the most common of which were attention deficit hyperactivity disorder (ADHD) and major depressive disorder (MDD). Regarding pharmacological treatments, six articles reported the use of more than one drug in the treatment of IGD, although only three articles reported results separately for different drugs. The most common drug used to treat IGD was bupropion or bupropion sustained release, which was used in six articles and among 26.9% (n=195) of participants across all studies. Other drugs used included a range of SSRIs, such as fluoxetine, escitalopram, and paroxetine (6 articles; n=169, 23.3%); methylphenidate (2 articles; n=106, 14.6%); and atomoxetine (1 article; n=40, 5.5%).
The most widely used instrument to measure gaming-related symptoms was the Young Internet Addiction Scale (YIAS) [27]. This scale, developed to analyze PIU, was used in eight of the 12 articles. The remaining four studies measured gaming-related symptoms using the Young’s Internet Addiction Test [28]. Four studies also examined time spent gaming as an outcome, which was measured via self-report and verified by collateral information (usually provided by a parent or spouse).
Of the 12 articles included in the present review, all reported reduced IGD symptoms from pre- to post-treatment across participants who received pharmacological treatment. Across all clinical trials, symptom reductions among participants who received treatment for IGD-related symptoms ranged from 15.4% to 51.4%. When stratifying analyses by specific drug, atomoxetine promoted an 18.3% symptom reduction, bupropion promoted symptom reductions ranging from 15.4% to 51.4%, SSRIs promoted symptom reductions ranging from 17.6% to 24.0%, and methylphenidate promoted symptom reductions ranging from 23.7% to 25.7%. Three articles focused on individuals with IGD and comorbid MDD [10,19,26], all reporting improvements in both MDD and IGD symptoms (n=124, 17.1%). Similarly, of the two articles that focused on individuals with ADHD who engaged in gaming [7,20], reductions in both ADHD and IGD symptoms were reported from pre- to post-treatment (n=146, 20.2%).
DISCUSSION
The aim of the present review was to synthesize the existing literature on pharmacological treatments for IGD. Overall, there is preliminary support that a wide range of pharmacological treatments may be effective in reducing symptoms of IGD and its associated comorbidities, including ADHD and depression. Although promising, the present review identified several gaps in the literature that warrant future address.
First, only 1.4% of the total sample were females. This differs markedly from the global demographic composition of video game players, in which women represent roughly 50% of gamers worldwide [29-31]. Furthermore, the rates of IGD in women are increasing, with current prevalence rates of 4%–5% [2], suggesting a need to examine gender differences in the efficacy of pharmacological treatment for IGD. Secondly, all studies included in the present review were conducted in South Korea. This may reduce the generalizability of the findings to people with IGD in other countries due to cross-cultural differences in IGD. For example, there may exist physiological differences among people of East Asian descent that may impact the efficacy of pharmacological treatments for IGD. Indeed, studies have reported on neurogenetic variance by ethnicity, particularly in dopamine polymorphism, which is implicated in addictive behaviors and depression symptoms [32,33].
Interestingly, most participants presented with psychiatric comorbidities, with only 24.0% of the total sample presenting exclusively with IGD. This finding is consistent with previous studies that have shown that IGD often presents with other mental health comorbidities [34]. The presence of comorbidities may render difficulties disentangling the intervention effects of pharmacological treatments on multiple co-occurring disorders. For example, in studies in which the samples were comprised of patients with IGD and ADHD who were treated with psychostimulants, it is difficult to determine whether the reductions in IGD symptoms were a direct result of the intervention, or were caused indirectly through reductions in ADHD symptoms [7,20]. The same challenge was presented in studies examining the treatment of IGD in the presence of comorbid depressive symptoms [10,19,24,26] and anxiety symptoms [24]. That said, the inclusion of people with IGD and mental health comorbidities better reflects clinical reality and likely provides more generalizable results. Future studies that investigate whether different pharmacological treatments have differential outcomes based on comorbid psychopathology would be highly informative.
Bupropion was the most frequently used drug in the treatment of IGD. Given that IGD is conceptualized as a behavioral addiction and most frequently co-occurs with depression, anxiety, and ADHD, this was not surprising, as bupropion is the only pharmacological treatment that has demonstrated efficacy in the treatment of smoking [35]; depressive disorders [36]; anxiety symptoms [37]; and ADHD [38]. These findings may illuminate the neurobiological mechanisms of action of bupropion in IGD, given the shared pathophysiology of dysregulated dopaminergic signaling across these conditions [39-41] and the role of bupropion in regulating dopamine release [42,43]. Furthermore, previous studies have also found increased default mode network (DMN) connectivity to correspond with dysregulated dopamingergic and norepinephrinergic signaling [44] as well as greater addictive behaviors [45,46], including impulsivity and risk-taking [47]. Importantly, two studies included in this review that investigated bupropion treatment of IGD observed decreases in functional connectivity within DMN [9,26] while one found decreased connectivity between the DMN and cognitive control network in people with IGD, which correlated with changes in IGD and MDD symptoms [26], potentially indicating a mechanism of action for bupropion in the treatment of IGD via regulation of dopamine and norepinephrine [43]. Comparatively, two studies investigated the efficacy of an SSRI in individuals with IGD [22,26]; one found decreased connectivity of the DMN, but not between the DMN and cognitive control network, and efficacy was lower relative to bupropion [26]. The other found normalization of absolute delta and theta power that correlated with reductions in IGD symptoms [22]. Normalization of resting state delta and theta waves may have implications for decreased DMN activity [48] and reflect improvements in inhibitory control [22]. However, this treatment did not correlate with reductions in co-occurring psychiatric symptoms. Thus, the co-occurring psychiatric symptoms presenting in IGD may have relevant implications for the preferred treatment method.
Worth noting, no studies in the current review examined pharmacological treatments targeting craving. Naltrexone is an anti-craving pharmacological intervention that is effective in the treatment of alcohol use disorder [49] and opioid use disorder [50], due to its antagonist actions in the mu-opioid system, thereby reducing rewarding effects of exogenous agonists such as alcohol and opioids. Some studies have investigated the use of naltrexone in behavioral addictions, with one small meta-analysis finding statistically significant improvements of naltrexone relative to placebo in treatment of gambling, kleptomania, trichotillomania, and impulsive-compulsive disorders [51]. In addition, one case-study reported reductions in IGD symptoms after naltrexone was administered to a 15-year old male [52]. However, research in the application of naltrexone to behavioral addictions is in its infancy, with existing studies having substantial heterogeneity, no randomized controlled trials of naltrexone on IGD, lack of long-term follow-ups, and limited empirical evidence on mechanisms of action. Nonetheless, the potential utility of naltrexone in treating IGD is a promising avenue of further study; long-term randomized clinical trials are needed to assess its effects.
This review is limited by several studies that did not provide a clear description of instruments used to measure primary and secondary outcomes. Most studies used the YIAS as an outcome measure, which may be problematic as the YIAS was originally developed for diagnosing PIU. As such, future pharmacological studies of IGD would benefit from including scales that have been developed specifically for the measurement of IGD, such as the Ten-Item Internet Gaming Disorder Test [53]. Further, the majority of the included studies were rated as having at least some concern of bias, with two rated as having serious concern. Potential bias in study findings limits adequate comparison of pharmacological treatments for IGD and may reduce confidence in the validity of the findings. Other methodological limitations include small sample sizes, inability to rule out regression to the mean in open label trials and lack of long follow up periods. Thus, future studies should ensure rigorous methodology.
An additional limitation is that studies rarely described the type(s) of games played by participants (e.g., role-playing, first-person shooter). Gaming preference is a relevant consideration as some game types, such as massively multiplayer online role playing games (MMORPG), are more strongly related to IGD than others, irrespective of player characteristics [54]. In addition, psychological profiles associated with game types may differ. For example MMORPGs are associated with high social anxiety, escapism, impulsivity, and sensation-seeking [55], which may not be the case for other game-types (e.g., offline computer games, arcade games). Given the heterogeneity in IGD presentation with respect to both game-level and player-level differences, future studies should examine the type of games to inform the selection of optimal pharmacological treatments for IGD.
Relatedly, examining the reasons why people play video games (i.e., gaming motivations) may also help to inform the selection of optimal treatments. For example, if a primary motivation for an individual with IGD is to regulate their affect due to symptoms of depression and anxiety, then antidepressant and anti-anxiety medications may be warranted. On the other hand, if individuals are motivated by need for achievement/advance (i.e., increase rank/pass levels), and purchase microtransactions such as loot boxes which operate on an intermittent reward schedule, then naltrexone may be of benefit. Furthermore, for individuals whose primary motivation is for social-relationships or high social anxiety then cognitive behavioral interventions may be the optimal treatment. However, we caution that further research is needed to examine whether gaming motives may have utility in selecting the most optimal treatments in IGD.
In summary, the literature examining pharmacological interventions is in its infancy. Although limited, results are promising and suggest that a wide array of pharmacological interventions may be leveraged in the treatment of IGD. Additional research is needed to further our understanding of pharmacological treatments for IGD. In particular, future studies using the gold standard methodology (i.e., double-blind randomized controlled trials), recruiting adequate sample sizes, controlling for psychiatric comorbidities, and consisting of more representative samples would better inform our understanding of the potential pharmacological treatments for this increasingly prevalent disorder. Moreover, an important direction for future research will be to explore heterogeneity in IGD presentation and treatment response to inform the selection of optimal treatments.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2022.0297.
Notes
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Rafael Richard Clorado de Sá, Hyoun Soo Kim, Hermano Tavares. Data curation: Sophie Coelho, Puneet Kaur Parmar, Hyoun Soo Kim. Formal analysis: Sophie Coelho, Puneet Kaur Parmar, Samantha Johnstone. Supervision: Hyoun Soo Kim, Hermano Tavares. Visualization: Sophie Coelho, Puneet Kaur Parmar. Writing—original draft: Rafael Richard Clorado de Sá, Sophie Coelho, Puneet Kaur Parmar, Samantha Johnstone. Writing—review & editing: Sophie Coelho, Puneet Kaur Parmar, Hyoun Soo Kim, Hermano Tavares.
Funding Statement
None
Acknowledgements
We would like to thank Ms. Kelly Dermody for their assistance with the search strategy.