1. Blazer DG, Hybels CF, Pieper CF. The association of depression and mortality in elderly persons: a case for multiple, independent pathways. J Gerontol A Biol Sci Med Sci 2001;56:M505-M509.


3. Park JH, Kim KW, Kim MH, Kim MD, Kim BJ, Kim SK, et al. A nationwide survey on the prevalence and risk factors of late life depression in South Korea. J Affect Disord 2012;138:34-40.


4. Koç Z. The investigation of factors that influence self-care agency and daily life activities among the elderly in the northern region of Turkey. Collegian 2015;22:251-258.


8. Allan CE, Valkanova V, Ebmeier KP. Depression in older people is underdiagnosed. Practitioner 2014;258:19-22.
9. Gallo JJ, Anthony JC, Muthén BO. Age differences in the symptoms of depression: a latent trait analysis. J Gerontol 1994;49:251-264.

10. Baldwin RC. Poor prognosis of depression in elderly people: causes and actions. Ann Med 2000;32:252-256.


11. Simpson S, Baldwin R, Jackson A, Burns A. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med 1998;28:1015-1026.


12. Prince MJ, Harwood RH, Thomas A, Mann AH. A prospective population-based cohort study of the effects of disablement and social milieu on the onset and maintenance of late-life depression. The Gospel Oak Project VII. Psychol Med 1998;28:337-350.


13. Sarin K, Punyaapriya P, Sethi S, Nagar I. Depression and hopelessness in institutionalized elderly: a societal concern. Open J Depress 2016;5:21


14. Ismail Z, Malick A, Smith EE, Schweizer T, Fischer C. Depression versus dementia: is this construct still relevant? Neurodegener Dis Manag 2014;4:119-126.


17. Santabarbara J, Sevil-Perez A, Olaya B, Gracia-Garcia P, Lopez-Anton R. Clinically relevant late-life depression as risk factor of dementia: a systematic review and meta-analysis of prospective cohort studies. Rev Neurol 2019;68:493-502.

19. Gellis ZD, McClive-Reed KP, Brown E. Treatments for depression in older persons with dementia. Treatments for depression in older persons with dementia. Ann Longterm Care 2009;17:29-36.


20. Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol 2013;9:91-121.


25. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566-572.


26. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-2414.

27. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, et al. Estimating the validity of the Korean version of expanded clinical dementia rating (CDR) scale. J Korean Neurol Assoc 2001;19:585-591.
28. Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982;139:1136-1139.


29. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Jeong Y, et al. The validity of the Korean version of Global Deterioration Scale. J Korean Neurol Assoc 2002;612-617.
30. Brink TL, Yesavage JA, Lum O, Heersema PH, Adey M, Rose TL. Screening tests for geriatric depression. Clin Gerontol 1982;1:37-43.

31. Jung IK, Kwak DI, Joe SH, Lee HS. A preliminary study on standardization of Korean form of geriatric depression scale (KGDS). J Korean Neurol Assoc 1998;340-351.
32. Epskamp S, Cramer AO, Waldorp LJ, Schmittmann VD, Borsboom D. qgraph: network visualizations of relationships in psychometric data. J Stat Softw 2012;48:1-18.
33. Van Borkulo CD, Borsboom D, Epskamp S, Blanken TF, Boschloo L, Schoevers RA, et al. A new method for constructing networks from binary data. Sci Rep 2014;4:5918


34. Fruchterman TM, Reingold EM. Graph drawing by force‐directed placement. Software: practice and experience 1991;21:1129-1164.

35. Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks: generalizing degree and shortest paths. Social networks 2010;32:245-251.

39. Júnior JASH, Fernandes ALAF, Medeiros AGAP, Vasconcelos CAC, Amorim LLL, Queiroga MFS, et al. Hopelessness in the elderly: a systematic review. MOJ Gerontology & Geriatrics 2018;3:273-278.
40. van Wanrooij LL, Borsboom D, Moll van Charante EP, Richard E, van Gool WA. A network approach on the relation between apathy and depression symptoms with dementia and functional disability. Int Psychogeriatr 2019;31:1655-1663.


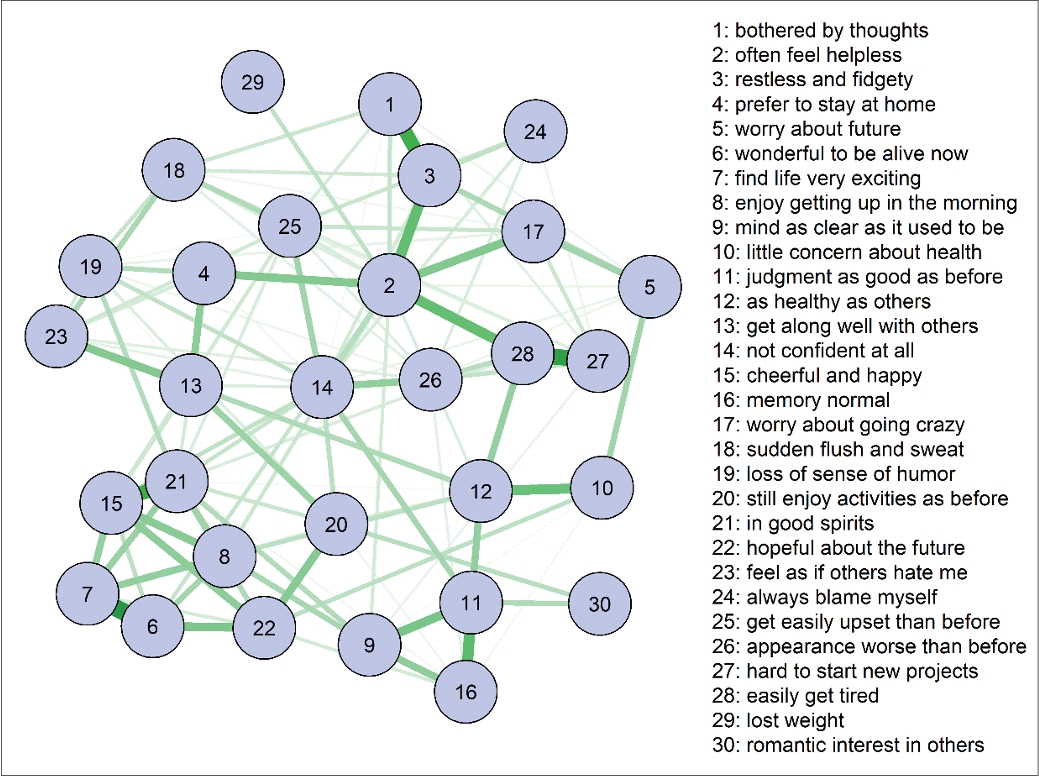
41. Kim D, Park SC, Kim IB, Kim E. Network analysis of central symptoms of depression in elderly cognitive disorder patients. J Korean Neurol Assoc 2022;61:38-44.


44. Abramson LY, Seligman ME, Teasdale JD. Learned helplessness in humans: critique and reformulation. J Abnorm Psychol 1978;87:49-74.


45. Targum SD, Nierenberg A. The complexity of “mixed” depression: a common clinical presentation. Innov Clin Neurosci 2011;8:38-42.


46. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003;58:249-265.


47. Spoletini I, Gianni W, Repetto L, Bria P, Caltagirone C, Bossu P, et al. Depression and cancer: an unexplored and unresolved emergent issue in elderly patients. Crit Rev Oncol Hematol 2008;65:143-155.


48. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275-281.


49. Volicer L, Frijters DH, Van der Steen JT. Relationship between symptoms of depression and agitation in nursing home residents with dementia. Int J Geriatr Psychiatry 2012;27:749-754.


50. Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry 2014;205:436-442.