|
 |
- Search
| Psychiatry Investig > Volume 21(5); 2024 > Article |
|
Abstract
Objective
Due to the high frequency of depressive symptoms associated with breast cancer, it is crucial to screen for depression in breast cancer patients. While numerous screening tools are available for depression in this population, there is a need for a brief and convenient tool to enhance clinical use. This study aims to investigate the psychometric properties of the Patient Health Questionnaire-9 (PHQ-9) in patients with breast cancer.
Methods
Patients with breast cancer (n=327) who visited the Breast Cancer Clinic were included in this study. The reliability of the PHQ-9 was analyzed by CronbachŌĆÖs ╬▒, and the construct validity of the PHQ-9 was explored by factor analysis. The concurrent validity of the PHQ-9 was evaluated by Pearson correlation analysis with the Hospital Anxiety and Depression Scale (HADS) and Perceived Stress Scale (PSS).
Results
The values of CronbachŌĆÖs ╬▒ ranged from 0.800 to 0.879 was acceptable. The exploratory factor analysis revealed that the one-factor model and two-factor model of the PHQ-9 explained 46% and 57% of the variance, respectively. The PHQ-9 were significantly correlated with those of HADS (r=0.702, p<0.001) and PSS (r=0.466, p<0.001). Consequently, the PHQ-9 demonstrated acceptable reliability and validity in breast cancer patients.
Breast cancer has shown a steady increase in survival rates, but its incidence is rising globally, making it a major cause of cancer-related death among the female population [1,2]. The high survival rate and high incidence ultimately result in a larger number of survivors, which also means an increase in the number of patients facing side effects [3]. This, in turn, poses a significant economic burden and health and social challenges in many countries [2]. Breast cancer is known to have a high prevalence of psychological comorbidities, with depression being particularly important for breast cancer patients [4]. Without proper care for the patients and their families, breast cancer patients can experience high levels of psychological stress, leading to increased physical burden. Depression has been identified as a predictor of increased hospitalization frequency, longer hospital stays, decreased quality of life, and reduced treatment compliance [5]. Moreover, depression is a crucial factor in predicting the delaying diagnosis of breast cancer patients [6] and is strongly associated with the suicide rate among them [7,8]. Ultimately, depression as a comorbidity in cancer patients is associated with poor prognosis and higher mortality rates [9]. Nonetheless, depression is likely underrecognized in cancer patients [10]. Early detection and the provision of appropriate treatment for psychological comorbidities are pivotal factors in enhancing both the survival rate and quality of life for breast cancer patients.
The reliability and validity of depression assessment tools used in psychiatry have been examined in breast cancer patients [11]. The 20-item Center for Epidemiologic Studies Depression Scale could be a good screening tool for depression in both the general population and breast cancer patients [12]. However, a brief assessment tool with fewer items may be more suitable for breast cancer patients with various medical conditions [13,14]. The 14-item Hospital Anxiety and Depression Scale (HADS) was developed to measure anxiety and depression levels in general hospital patients [15]. The HADS demonstrated excellent psychometric properties in patients who underwent breast surgery and breast cancer survivors [16-18]. Therefore, the HADS is widely used as a standard screening tool for breast cancer patients [16-18]. Alternatively, the Distress Thermometer (DT), a single-item question, is sometimes used as a screening tool [19]. The DT is a visual analog scale that rates emotional distress from 0 (no distress) to 10 (extreme distress) using a thermometer-like image. Several studies have shown that the DT has good psychometric properties despite one-item measure, but its low specificity is a limitation [20-23].
The 9-item Patient Health Questionnaire-9 (PHQ-9) is another brief, reliable and valid screening tool for depression in the general population or clinical samples [24-26]. The PHQ-9 has been used in studies with breast cancer patients [27,28], but there has been only one preliminarily study that examined the reliability and validity of the Portuguese version of the PHQ-9 in 49 breast cancer patients [29]. Moreover, the construct validity of PHQ-9 in breast cancer patients has not yet been reported. The pattern of depression measured by the PHQ-9 might be different in cancer patients, compared to general population [30]. Although the PHQ-9 was validated to screen for depression in the general population, the reliability and validity of the PHQ-9 may vary depending on the characteristics of the group when considering the heterogeneity of depression. In particular, breast cancer, unlike other cancers, was associated with low body image, decreased attractiveness, and impaired femininity [31]. Therefore, the aim of this study was to investigate the reliability and validity of the PHQ-9 in patients with breast cancer.
Patients attending the Breast Cancer Clinic in Pusan National University Hospital from February 2021 to December 2021 were participated in this study. Inclusion criteria comprised patients diagnosed with stage 0-III, while individuals more than 5 years post-breast surgery and those who declined psychiatric evaluation were excluded. A total of 327 patients were included. This study was approved by the Institutional Review Board at Pusan National University Hospital (PNUH IRB: No. 2301-004-122). Informed consent was obtained form all participants.
The reliability and validity of the PHQ-9 as a screening tool for depression in patients attending a breast cancer clinic were investigated cross-sectionally. Demographic variables were assessed using electronic medical record.
PHQ-9 consisting of 9 items was developed to screen the high-risk group of depression in the general population [32]. The PHQ-9 is a brief self-report questionnaire. Previous studies on psychometric properties of PHQ-9 in the general population reported acceptable reliability and validity [32]. When the score of the PHQ-9 was less than 5, it was considered normal. A score of 5-9 was considered mild depression, and a score of 10-14 was considered moderate depression. A score of 15-19 was considered moderate-severe depression, and a score of 20 or more was considered severe depression [33]. The Korean version of PHQ-9 showed acceptable reliability and validity to screen high-risk groups of depression [34].
The HADS, developed in 1983 by Zigmond and Snaith [15], consists of a 14-item scale, with seven items related to anxiety and seven to depression. The scale was designed to assess the degree of anxiety and depression experienced by patients awaiting medical treatment in general hospitals within a short timeframe. According to the developers, this scale proves beneficial in evaluating the levels of anxiety and depression, as well as changes in emotional states, for patients visiting general hospitals. It is also a measure of standardization and feasibility studies conducted in various studies [35,36].
Perceived Stress Scale (PSS) was a self-rated questionnaire to measure the level of stress perception [37]. The questions about positive and negative perceptions on stressful situations ranged from 0 to 40 scores. A higher score represented more perceived stress. The Korean version of PSS showed good psychometric properties [38].
Sociodemographic and clinicopathological characteristics in patients with breast cancer were presented as mean and standard deviation. The reliability of PHQ-9 was analyzed by CronbachŌĆÖs ╬▒, an indicator of internal consistency. The construct validity of PHQ-9 was examined through exploratory factor analysis with principal components analysis. Varimax rotation was applied to analyze the construct validity of the two-factor model. The determination of the number of factors was analyzed using scree plots based on eigenvalues. Concurrent validity of PHQ-9 was assessed using HADS, and PSS and this was analyzed through Pearson correlation analysis. Statistical analysis was conducted using SPSS 22.0 (IBM Corp., Armonk, NY, USA). Scatter plots were generated using the corrplot package in R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
The demographic variables of the subjects in this study are shown in Table 1. The mean age of patients with breast cancer was 48.7(┬▒6.4). In terms of marital status, 269 individuals (82.3%) were married, while 54 persons (16.5%) were unmarried. Among the total subjects, 65 patients (19.9%) were in the menopausal age group.
The distribution of cancer stages was as follows: stage 0 in 48 patients (14.7%), stage I in 136 patients (41.6%), stage II in 126 patients (38.5%), and stage III in 17 patients (5.2%). Regarding cancer histological findings, 284 patients (86.9%) had ductal type, 17 patients (5.2%) had lobular type, 5 patients (1.5%) had mixed type, and 8 patients (2.4%) had an unknown type. In terms of surgery, 203 patients (62.1%) underwent partial resection, 36 patients (11.0%) had total resection without reconstruction, and 88 patients (26.9%) had total resection with reconstruction. Among the patients, 263 (80.4%) received anti-hormone therapy, 221 (67.6%) underwent radiation therapy, 158 (48.3%) received adjuvant chemotherapy, and 34 (10.4%) received targeted therapy.
The depression scores, measured by PHQ-9, indicated normal levels in 47.7% (156/327) of participants, mild depression in 32.4% (106/327), moderate depression in 16.8% (55/327), and moderate to severe depression in 2.4% (8/327). Severe depression was observed in 0.6% (2/327) of the participants.
The value of CronbachŌĆÖs ╬▒ was 0.840. The 95% confidence interval for CronbachŌĆÖs ╬▒ ranges from 0.800 to 0.879.
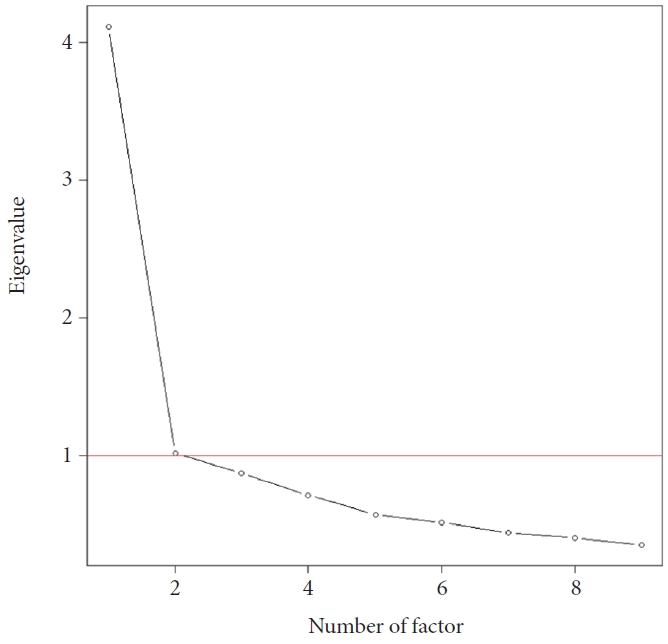
In the scree plot based on the eigenvalue as shown in Figure 1, an eigenvalue value of 1 or more was investigated as a factor of two. In the one-factor model, a proportional variation was investigated as 0.46. In the two-factor model, the proportional variation of factor 1 was 0.38, the proportional variation of factor 2 was 0.19, and the cumulative variation of factor 1 and 2 was 0.57.
The factor loadings for each item in both the one-factor and two-factor models are presented in Table 2. In the one-factor model, item 8 displayed the lowest factor loading at 0.47, while the other items had factor loadings ranging from 0.65 to 0.75. In the two-factor model, most items exhibited high factor loadings, ranging from 0.63 to 0.75, except for items 7 and 8 in the one-factor case. Specifically, in the two-factor model, item 7 demonstrated a factor loading of 0.74, and item 8 showed the highest factor loading at 0.88.
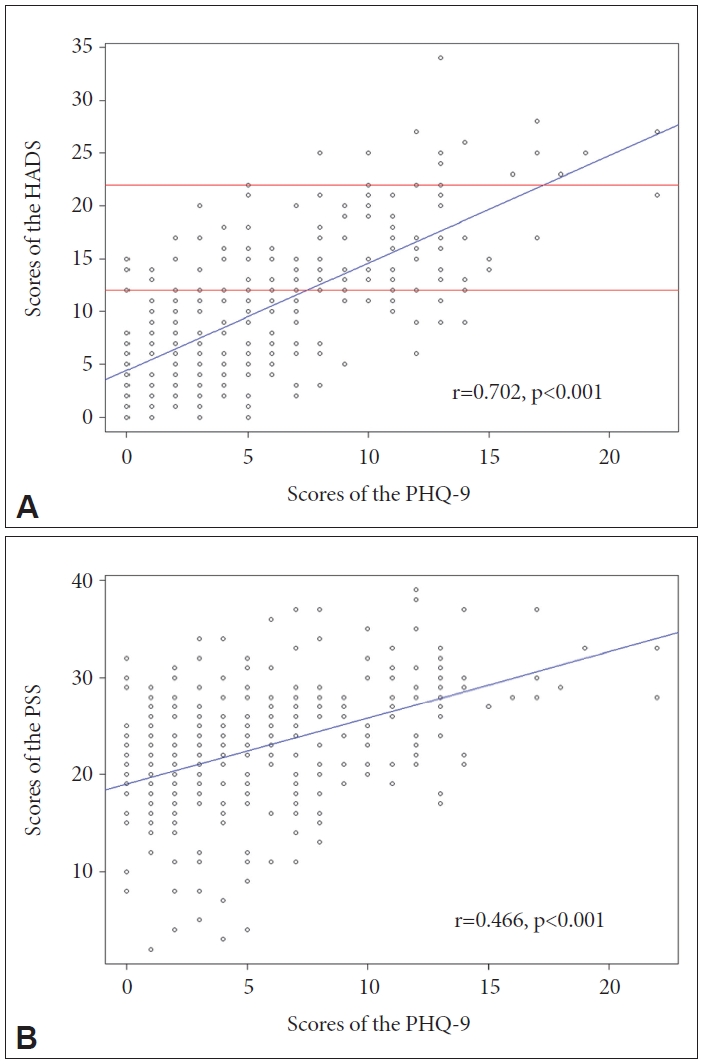
As depicted in Figure 2A, the PHQ-9 exhibited a strong positive correlation with the HADS total score (r=0.702, p<0.001). Furthermore, a significant positive correlation was observed in both the anxiety subtotal score (r=0.627, p<0.001) and depression subtotal score (r=0.670, p<0.001). Additionally, the PHQ-9 demonstrated a high positive correlation with the PSS total score, as illustrated in Figure 2B (r=0.466, p<0.001).
The Korean version of the PHQ-9 demonstrated acceptable reliability and validity in patients with breast cancer. The PHQ-9 is widely used as a self-report questionnaire. This scale tried to screen the depression in various samples such as the general population, primary care patients, and cancer patients [24]. Although the PHQ-9 had been used in studies with breast cancer patients [27,28,39], only one preliminary study reported the reliability and validity of the Portuguese version of the PHQ-9 in 49 breast cancer patients [29]. However, the construct validity of the PHQ-9 in breast cancer patients has not been reported yet. This study suggests that the PHQ-9 could serve as a reliable and valid screening tool for patients with breast cancer. The CronbachŌĆÖs ╬▒ of PHQ-9 in patients with breast cancer was 0.840. In general, CronbachŌĆÖs ╬▒ is preferably 0.70 or above is good, 0.80 or above is better, and 0.90 or above is best [40]. As the previous study reported a CronbachŌĆÖs ╬▒ of 0.82 [29], the internal consistency of PHQ-9 in this study was found to be good.
The exploratory factor analysis of the PHQ-9 in breast cancer patients showed good construct validity, as shown in Table 2. These findings were mostly consistent with previous validity studies in various samples [24]. The one-factor model revealed a proportional variation of 0.46. In this model, factor loadings ranged from 0.472 to 0.754. Although factor loading was the lowest in item 8 at 0.472, most of the items showed favorable factor loadings of 0.6 or more [41]. The one-factor model of the PHQ-9 demonstrated acceptable construct validity when used with breast cancer patients. Meanwhile, the cumulative variation in the two-factor model increased to 0.57. In the two-factor model, factor loadings were consistently high, ranging from 0.63 to 0.75, except for items 7 and 8 in the one-factor case. In the two-factor model, item 7 showed a factor loading of 0.74, and item 8 demonstrated the highest factor loading at 0.88. While most studies predominantly suggest using the one-factor model [24], some studies report that the two-factor model may be a better scale [30,42-45]. Considering that depressive symptoms can be heterogeneous, factors of depressive symptoms may vary according to the characteristics such as sex, race, education level [43,44]. As breast cancer primarily affects female patients, women bear the brunt of the challenges posed by this specific cancer [46,47]. Moreover, the survival period for breast cancer is typically long, leading to various concerns about life [48]. Particularly, young breast cancer patients face substantial challenges, particularly relating to self-image and parenting concerns [49]. Given these considerations, it is unsurprising that patients with advanced breast cancer may experience heightened levels of agitation [50]. On the other hand memory impairment and diminished concentration are also frequently reported symptoms among breast cancer patients [51]. Therefore, it is crucial to examine symptom patterns in breast cancer patients to understand how psychomotor changes and cognitive symptoms present distinctly from other depressive symptoms. Future studies should also consider the validity of the two-factor model when using the PHQ-9 to assess depression in breast cancer patient group.
In this study, moderate to severe depression with a PHQ-9 score of 10 or higher was found to be 19.8%. Despite variances in the criteria across different studies, the prevalence of depression identified in this research aligns with the findings of other studies [52,53]. Furthermore, the concurrent validity of the PHQ-9 was demonstrated through high correlations identified in the analysis with both the HADS and the PSS. In addition, a preliminary validation study conducted among Portuguese breast cancer patients also reported significant correlations between the PHQ-9 and the anxiety and depression subscales of the HADS [29]. The HADS is widely recognized as an effective tool for assessing depression in breast cancer patients. Given the high correlation observed between the PHQ-9 and HADS in this study, it can be considered as having concurrent validity. Previous studies utilizing the HADS for depression screening in breast cancer patients have reported cutoff scores ranging from 12 to 22 [54,55]. Based on the results of the correlation analysis, applying these cutoff scores to the PHQ-9 suggests an estimated range of 8 to 17 points (Figure 2A). However, further research is needed to determine the appropriate cutoff score for screening depression using the PHQ-9 specifically in breast cancer patients. Furthermore, there was a notable association between depressive symptoms and perceived stress. As breast cancer patients experiencing higher levels of stress during treatment also tended to exhibit more severe depression, it becomes essential to assess whether the PHQ-9 accurately reflects the degree of stress. The strong correlation observed between the PSS and PHQ-9 indicates that the PHQ-9 might effectively capture the link between stress and depressive symptoms among breast cancer patients. Based on these findings, it is evident that the PHQ-9 serves as a reliable assessment tool for evaluating depressive symptoms and effectively capturing the impact of stress among individuals.
This study has several limitations that should be acknowledged. Firstly, this study primarily focused on exploring the construct validity of the PHQ-9 through exploratory factor analysis. Future research should aim to confirm whether the one-factor model or two-factor model of the PHQ-9 is more optimal. Secondly, the concurrent validity was assessed solely with the HADS and the PSS, limiting the scope of the validation process. Future studies should investigate the concurrent validity of the PHQ-9 in relation to various clinical prognoses. Thirdly, this study did not establish an optimal cutoff score for screening depression in breast cancer patients. Future research should focus on identifying an appropriate cutoff score for depression screening in this specific patient population. Lastly, the study did not differentiate between different cancer types, stages, and treatment phases of breast cancer. The findings may vary across distinct clinical situations, warranting further investigation into the reliability and validity of the PHQ-9 based on the specific cancer type, stage, and treatment phase.
In conclusion, this study establishes the potential of the PHQ-9 as a reliable and valid screening tool for depression in breast cancer patients. Its brevity, consisting of only 9 items, makes it a convenient questionnaire for implementation in breast cancer clinics. However, to confirm its structural validity, further examination through confirmatory factor analysis is necessary. Additionally, exploring the clinical usefulness of the PHQ-9 by assessing various aspects of concurrent validity is warranted. Furthermore, it is crucial to evaluate to see if the PHQ-9 remains reliable and valid in different clinical scenarios.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Heeseung Park, Eunsoo Moon, Taewoo Kang. Data curation: Heeseung Park. Formal analysis: Kyungwon Kim, Hyunju Lim. Investigation: Heeseung Park, Eunsoo Moon, Taewoo Kang. Methodology: Heeseung Park, Kyungwon Kim, Eunsoo Moon, Hwagyu Suh, Taewoo Kang. Validation: Hwagyu Suh, Taewoo Kang. Visualization: Kyungwon Kim. WritingŌĆöoriginal draft: Heeseung Park, Eunsoo Moon. WritingŌĆöreview & editing: all authors.
Funding Statement
None
Figure┬Ā2.
Scatter plots between the scores of PHQ-9 and those of HADS and PSS. A: Showed the scatter plot and best fit (blue line) between PHQ-9 and HADS. Also, the cutoffs (red line) ranged from 12 to 22 scores of the HADS estimated the cutoff of the PHQ-9. B: Showed the scatter plot and best fit (blue line) between PHQ-9 and PSS. PHQ-9, the Patient Health Questionnaire-9; HADS, Hospital Anxiety and Depression Scale; PSS, Perceived Stress Scale.
Table┬Ā1.
Sociodemographic and clinicopathological characteristics in patients with breast cancer
| Characteristics | Value (N=327) |
|---|---|
| Age (yr) | 48.7┬▒6.4 |
| Education year | 13.7┬▒3.3* |
| Marital status | |
| ŌĆāMarried | 269 (82.3) |
| ŌĆāUnmarried | 54 (16.5) |
| ŌĆāDivorced | 4 (1.2) |
| Postmenopause | 65 (19.9) |
| Stage | |
| ŌĆā0 | 48 (14.7) |
| ŌĆāI | 136 (41.6) |
| ŌĆāII | 126 (38.5) |
| ŌĆāIII | 17 (5.2) |
| Histology | |
| ŌĆāDuctal | 284 (86.9) |
| ŌĆāLobular | 17 (5.2) |
| ŌĆāMixed | 5 (1.5) |
| ŌĆāOthers | 13 (4.0) |
| ŌĆāUnknown | 8 (2.4) |
| Surgery type | |
| ŌĆāPartial resection | 203 (62.1) |
| ŌĆāTotal without reconstruction | 36 (11.0) |
| ŌĆāTotal with reconstruction | 88 (26.9) |
| Adjuvant treatmentŌĆĀ | |
| ŌĆāAntihormonal treatment | 263 (80.4) |
| ŌĆāRadiation | 221 (67.6) |
| ŌĆāChemotherapy | 158 (48.3) |
| ŌĆāTarget therapy | 34 (10.4) |
| PHQ-9 total score | 5.8┬▒4.4 |
| HADS total score | 10.1┬▒6.4 |
| PSS total score | 22.8┬▒6.5 |
Table┬Ā2.
The results of exploratory factor analysis on PHQ-9
REFERENCES
1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021;127:3029-3030.



2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-249.



3. Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol 2008;26:768-777.


4. Kissane DW, Grabsch B, Love A, Clarke DM, Bloch S, Smith GC. Psychiatric disorder in women with early stage and advanced breast cancer: a comparative analysis. Aust N Z J Psychiatry 2004;38:320-326.


5. Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol 2002;57:41-49.

6. Trinca F, Infante P, Dinis R, In├Īcio M, Bravo E, Caravana J, et al. Depression and quality of life in patients with breast cancer undergoing chemotherapy and monoclonal antibodies. Ecancermedicalscience 2019;13:937



7. Desai MM, Bruce ML, Kasl SV. The effects of major depression and phobia on stage at diagnosis of breast cancer. Int J Psychiatry Med 1999;29:29-45.



8. Schairer C, Brown LM, Chen BE, Howard R, Lynch CF, Hall P, et al. Suicide after breast cancer: an international population-based study of 723,810 women. J Natl Cancer Inst 2006;98:1416-1419.


9. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer 2009;115:5349-5361.


10. Hardman A, Maguire P, Crowther D. The recognition of psychiatric morbidity on a medical oncology ward. J Psychosom Res 1989;33:235-239.


11. Park H, Kim KE, Moon E, Kang T. Psychometric properties of assessment tools for depression, anxiety, distress, and psychological problems in breast cancer patients: a systematic review. Psychiatry Investig 2023;20:395-407.




12. Ashing-Giwa K, Rosales M. A cross-cultural validation of patient-reported outcomes measures: a study of breast cancers survivors. Qual Life Res 2013;22:295-308.



13. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst 2009;101:1464-1488.



14. Stafford L, Judd F, Gibson P, Komiti A, Mann GB, Quinn M. Screening for depression and anxiety in women with breast and gynaecologic cancer: course and prevalence of morbidity over 12 months. Psychooncology 2013;22:2071-2078.


15. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-370.


16. Saboonchi F, Wennman-Larsen A, Alexanderson K, Petersson LM. Examination of the construct validity of the Swedish version of hospital anxiety and depression scale in breast cancer patients. Qual Life Res 2013;22:2849-2856.



17. Tomljenovi─ć H, Murgi─ć J, Matija┼Ī M, Jazvi─ć M, Brozi─ć JM, Kirac I, et al. Hospital anxiety and depression scale: psychometric validation on a sample of Croatian breast cancer patients. Libri Oncol 2021;49:101-102.
18. Hajian-Tilaki K, Hajian-Tilaki E. Factor structure and reliability of Persian version of hospital anxiety and depression scale in patients with breast cancer survivors. Health Qual Life Outcomes 2020;18:176




19. Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer 1998;82:1904-1908.


20. Bidstrup PE, Mertz BG, Dalton SO, Deltour I, Kroman N, Kehlet H, et al. Accuracy of the Danish version of the ŌĆśdistress thermometerŌĆÖ. Psychooncology 2012;21:436-443.



21. Yong HW, Zubaidah J, Saidi M, Zailina H. Validation of Malaysian translated distress thermometer with problem checklist among the breast cancer survivors in Malaysia. Asian J Psychiatr 2012;5:38-42.


22. Iskandarsyah A, de Klerk C, Suardi DR, Soemitro MP, Sadarjoen SS, Passchier J. The distress thermometer and its validity: a first psychometric study in Indonesian women with breast cancer. PLoS One 2013;8:e56353.



23. Civilotti C, Acquadro Maran D, Santagata F, Varetto A, Stanizzo MR. The use of the distress thermometer and the hospital anxiety and depression scale for screening of anxiety and depression in Italian women newly diagnosed with breast cancer. Support Care Cancer 2020;28:4997-5004.



24. Lamela D, Soreira C, Matos P, Morais A. Systematic review of the factor structure and measurement invariance of the patient health questionnaire-9 (PHQ-9) and validation of the Portuguese version in community settings. J Affect Disord 2020;276:220-233.


25. Suh H, Moon E, Park JM, Lee BD, Lee YM, Jeong HJ, et al. A validation study of mental health monitoring through a mobile application. Psychiatry Investig 2023;20:575-580.




26. Lim HJ, Moon E, Kim K, Suh H, Park J, Kim DR, et al. Cluster analysis on the mental health states in a community sample of young women during pre-pregnancy, pregnancy, or the postpartum period. Psychiatry Investig 2023;20:445-451.




27. Kim J, Lim S, Min YH, Shin YW, Lee B, Sohn G, et al. Depression screening using daily mental-health ratings from a smartphone application for breast cancer patients. J Med Internet Res 2016;18:e216



28. Ganz PA, Bower JE, Partridge AH, Wolff AC, Thorner ED, Joffe H, et al. Screening for depression in younger breast cancer survivors: outcomes from use of the 9-item patient health questionnaire. JNCI Cancer Spectr 2021;5:pkab017




29. Torres A, Pereira A, Monteiro S, Albuquerque E. Preliminary psychometric characteristics of the Portuguese version of patient health questionnaire 9 (PHQ-9) in a sample of Portuguese breast cancer women. Eur Psychiatry 2013;28:1

30. Hinz A, Mehnert A, Kocalevent RD, Br├żhler E, Forkmann T, Singer S, et al. Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry 2016;16:22



31. Begovic-Juhant A, Chmielewski A, Iwuagwu S, Chapman LA. Impact of body image on depression and quality of life among women with breast cancer. J Psychosoc Oncol 2012;30:446-460.


32. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-613.



34. An JY, Seo ER, Lim KH, Shin JH, Kim JB. [Standardization of the Korean version of screening tool for depression(patient health questionnaire-9, PHQ-9)]. J Korean Soc Biol Ther Psychiatry 2013;19:47-56. Korean.
35. Lisspers J, Nygren A, S├Čderman E. Hospital anxiety and depression scale (HAD): some psychometric data for a Swedish sample. Acta Psychiatr Scand 1997;96:281-286.


36. Malasi TH, Mirza IA, el-Islam MF. Validation of the hospital anxiety and depression scale in Arab patients. Acta Psychiatr Scand 1991;84:323-326.


37. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385-396.


38. Lee J, Shin C, Ko YH, Lim J, Joe SH, Kim S, et al. [The reliability and validity studies of the Korean version of the perceived stress scale]. Korean J Psychom Med 2012;20:127-134. Korean.
39. Li Y, Liu H, Sun Y, Li J, Chen Y, Zhang X, et al. Characteristics and subtypes of depressive symptoms in Chinese female breast cancer patients of different ages: a cross-sectional study. AIMS Public Health 2021;8:691-703.



40. Nunnally JC, Bernstein IH. Psychometric theory (3rd ed). New York: McGraw-Hill; 1994.
41. MacCallum RC, Widaman KF, Preacher KJ, Hong S. Sample size in factor analysis: the role of model error. Multivariate Behav Res 2001;36:611-637.


42. Chilcot J, Rayner L, Lee W, Price A, Goodwin L, Monroe B, et al. The factor structure of the PHQ-9 in palliative care. J Psychosom Res 2013;75:60-64.


43. Patel JS, Oh Y, Rand KL, Wu W, Cyders MA, Kroenke K, et al. Measurement invariance of the patient health questionnaire-9 (PHQ-9) depression screener in U.S. adults across sex, race/ethnicity, and education level: NHANES 2005-2016. Depress Anxiety 2019;36:813-823.




44. Petersen JJ, Paulitsch MA, Hartig J, Mergenthal K, Gerlach FM, Gensichen J. Factor structure and measurement invariance of the patient health questionnaire-9 for female and male primary care patients with major depression in Germany. J Affect Disord 2015;170:138-142.


45. Arnold SRC, Uljarevi─ć M, Hwang YI, Richdale AL, Trollor JN, Lawson LP. Brief report: psychometric properties of the patient health questionaire-9 (PHQ-9) in autistic adults. J Autism Dev Disord 2020;50:2217-2225.



46. Monta├▒├®s-Muro P, Mart├Łnez-Tom├® M, Garc├Ła-Manzano G. Psychosocial care needs of women with breast cancer: body image, self-esteem, optimism, and sexual performance and satisfaction. Health Soc Work 2023;48:115-123.



47. Tang ELS, Sin PY, Chen JJC, Chan MYP, Seah MDW, Lu SQ, et al. Understanding the psychosocial needs of women who present with advanced breast cancer. Ann Acad Med Singap 2020;49:990-995.


48. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer 2008;112(11 Suppl):2577-2592.


49. Yfantis A, Intas G, Tolia M, Nikolaou M, Tsoukalas N, Lymperi M, et al. Health-related quality of life of young women with breast cancer. Review of the literature. J BUON 2018;23:1-6.
50. Cherny NI, Paluch-Shimon S, Berner-Wygoda Y. Palliative care: needs of advanced breast cancer patients. Breast Cancer (Dove Med Press) 2018;10:231-243.




51. Joly F, Lange M, Dos Santos M, Vaz-Luis I, Di Meglio A. Long-term fatigue and cognitive disorders in breast cancer survivors. Cancers (Basel) 2019;11:1896



52. Aapro M, Cull A. Depression in breast cancer patients: the need for treatment. Ann Oncol 1999;10:627-636.


53. Casavilca-Zambrano S, Custodio N, Liendo-Picoaga R, Cancino-Maldonado K, Esenarro L, Montesinos R, et al. Depression in women with a diagnosis of breast cancer. Prevalence of symptoms of depression in Peruvian women with early breast cancer and related sociodemographic factors. Semin Oncol 2020;47:293-301.