|
 |
- Search
| Psychiatry Investig > Volume 13(4); 2016 > Article |
Abstract
Objective
To determine the predictive validity of some of the commonly employed models of mania and depression using standard drugs i.e. lithium (70 mg/kg) and lamotrigine (5 mg/kg) in male Wistar rats.
Methods
The depression facet of bipolar disorder was evaluated using forced swim test, tail suspension test, and chronic mild stress test. The models used to evaluate the mania facet of bipolar disorder were isolation-induced aggression test, saccharine preference test, and morphine-sensitized hyperlocomotion test.
Results
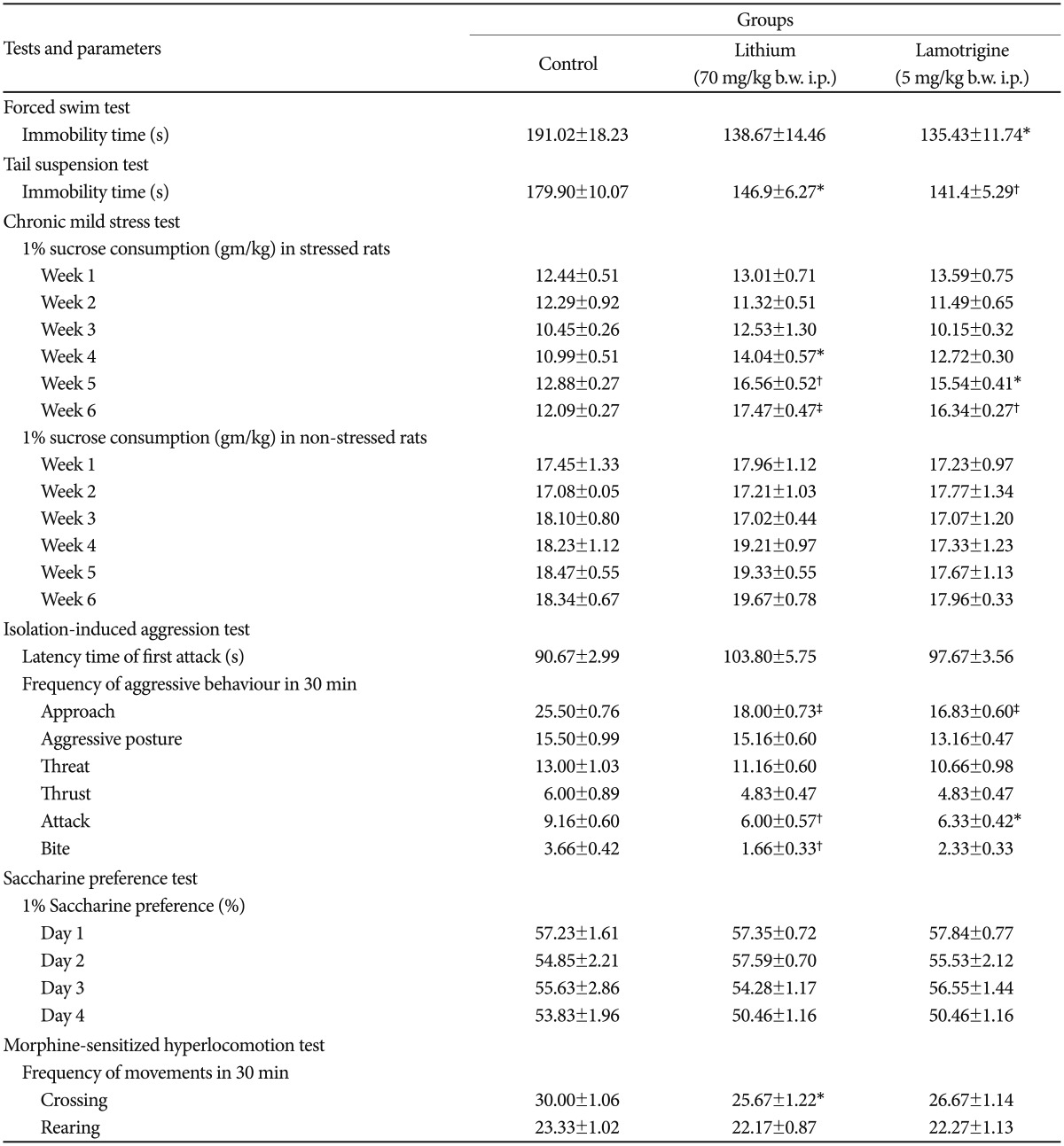
The immobility time was significantly (p<0.05) reduced by lamotrigine in the tail suspension test and the forced swim test, while lithium caused significant (p<0.05) reduction only in the tail suspension test. Rats exposed to chronic mild stress showed the maximal increment of 1% sucrose consumption at the 3rd week of treatment in both the lithium (p<0.001) and lamotrigine (p<0.01) groups. In the isolation-induced aggression test, the aggressive behaviour of rats was significantly reduced by both lithium [approach (p<0.001), attack (p<0.01), and bite (p<0.01)] and lamotrigine [approach (p<0.001), and attack (p<0.05)]. Neither of the drugs were effective in the saccharine preference test. Only lithium was able to significantly (p<0.05) reduce the crossing parameter in morphine-sensitized rats.
Bipolar disorder (BD) is a major mental health problem with a lifetime prevalence rate of 2.2% worldwide, and is associated with considerable morbidity, mortality, and diminished quality of life.1 Some of the drugs approved for the treatment of BD are lithium, valproic acid, carbamazepine, lamotrigine, aripiprazole, chlorpromazine, olanzapine, quetiapine, risperidone, and ziprasidone.2 The present drug therapy available for BD improves the symptoms and reduces the burden of the disease, yet there is a failure to complete recovery, hence there is a pressing need for new effective medications.34 One of the major hurdles in the development of new drugs for BD is the absence of suitable animal models.56
Most of the present animal models for BD, developed by pharmacological/environmental/behavioural/genetic manipulations, target either the mania or the depression facet of the disease and have numerous limitations.5 One of the solutions suggested for improving the present scenario is by developing a battery of models, targeting different facets of the disorder, rather than relying on one particular model. A leverage of the battery-based, approach is that each model may be only partially valid when used alone but the combination of a few models may result in strong validity.7 Previous studies in rodents have shown that lithium is effective in reducing aggression, and also inhibits depressive-like behaviours.89 Lamotrigine has demonstrated an antidepressant activity in previous animal studies and its effect in aggression/mania is also sparsely documented.1011 Little attempt has been made to incorporate the above findings towards a battery-based approach.
Any new model proposed for BD should have construct, predictive, and face validities.12 Construct validity refers to the commonalties between the mechanism of the model and of the human disorder.13 Face validity indicates that a model reproduces significant anatomical, biochemical, neuropathological, or behavioural features of a human disease.6 Predictive validity signifies that an animal phenotype responds to treatments in a model in a similar way as in humans.14 The aim of the present study was to determine the predictive validity of some of the commonly employed animal models used for mania and depression, which are the two most important facets of BD, using lithium and lamotrigine as standard drugs. The authors have proposed that the models showing the highest predictive validity in mania and depression should be a part of a battery of tests used for evaluating novel mood stabilizers.
Male Wistar rats, weighing 200-250 g, 12-16 weeks of age at the beginning of the experiment, were obtained from the Animal House of Vardhman Mahavir Medical College and Safdarjung Hospital (VMMC and SJH), New Delhi. Animals were acclimatized to laboratory conditions before the start of the study. The animals were weighed, marked, and housed in groups/alone, depending on the test employed, under controlled conditions of 24±2℃, 12 h light/dark cycle, with food and water ad libitum. Experimental procedures and protocols used in the study were approved by the 'Institutional Animal Ethics Committee' of VMMC and SJH, and conform to the "Guidelines for care and use of animals in scientific research" (Indian National Science Academy 1998, Revised 2000). The rats were not used in more than one test.
Lithium and lamotrigine were gifted by Torrent Pharmaceuticals Limited, New Delhi, India. Morphine was procured from Sigma-Aldrich Corporation, St. Louis, MO, USA.
The rats were divided into 3 groups (n=6), each receiving 0.5 mL intraperitoneal (i.p.) normal saline, 70 mg/kg body weight (b.w.) i.p. lithium, and 5 mg/kg b.w. i.p. lamotrigine, respectively. The test was carried out according to the method described earlier, in a clear glass tank (25×25×60 cm), containing 39 cm clean water (26℃ temperature), where rats cannot touch the bottom of the tank or escape.15 Swimming sessions were conducted on the 1st day for 15 min as habituation and on the 2nd day for 6 min as the test session. The behaviour of the rats was videotaped and the immobility time was recorded for the test session. Mobility of the rats was defined as any movements other than those necessary to balance the body and keep the head above the water.
The test was carried out according to the method described earlier.16 Rats were divided into 3 groups (n=6) and were administered 0.5 mL i.p. normal saline, 70 mg/kg b.w. i.p. lithium, and 5 mg/kg b.w. i.p. lamotrigine, respectively. The rats were suspended 50 cm above the floor by an adhesive tape, applied at 1 cm from the tip of their tails, for 6 min. The rats became immobile after some amount of struggle. The behaviour and immobility time of the rats was video recorded. Rats were considered immobile when they were completely motionless.
Rats were trained for the consumption of 1% sucrose solution and distilled water. Chronic mild stress was induced in 18 rats for a duration of 6 weeks by 4 h of food and water deprivation, continuous lighting, cage tilt (30°), paired housing, soiled cage, and exposure to reduced temperature (10℃), as described earlier.1718 After 3 weeks of chronic mild stress, the rats were divided into 3 groups (n=6) and were administered a daily dose of 0.5 mL i.p. normal saline, 70 mg/kg b.w i.p. lithium, and 5 mg/kg b.w. i.p. lamotrigine, respectively. Six rats were housed in a group and their fluid intake of sucrose/distilled water was measured on one specified day (Tuesday), of all the 6 weeks of the experiment, by reweighing preweighed bottles, 60 min after drug administration. In addition to the 3 groups mentioned above, there were 3 more groups (n=6) of non-stressed rats receiving the same doses of normal saline, lithium and lamotrigine and evaluated similarly.
The test was conducted according to the method described earlier.1920 Rats were kept in isolation for 3 weeks. The aggressive behaviour of the isolated rat was assessed against a male rat (similar in weight to that of the isolated rat, and accustomed to living in a group and put into the cage of an isolated rat for 5 min). Isolated rats not exhibiting aggressive behaviour were excluded from the test. The aggressive rats were randomly distributed into 3 groups (n=6) and were treated with a daily dose of 0.5 mL i.p. normal saline, 70 mg/kg b.w. i.p. lithium, and 5 mg/kg b.w. i.p. lamotrigine, respectively, for 3 consecutive days. One hour after the last dose, a non-aggressive rat was introduced to a cage containing an aggressive rat. The latency time of the first attack was recorded and the frequency of each of the following behaviours listed below was recorded for a period of 30 min: approach (a movement towards the other rat), aggressive posture (orients itself at right angle to the other rat), threat (head movement towards the other rat), thrust (whole body movement towards the other rat), attack (rapid movement towards the other rat), and bite (biting the other rat). In case of a harmful attack, the aggressive rat was withdrawn from the cage.
A modified saccharine solution preference test was conducted as described earlier.21 Saccharine solution preference was calculated as percentage of saccharine solution out of total liquid consumption. The rats were provided with a bottle of 1% saccharine solution, in addition to regular supply of water for 7 days. Rats with a preference for saccharine solution(>50% of the total liquid consumption) were selected for the study and divided into 3 groups (n=6) and injected with a daily dose of 0.5 mL i.p. normal saline, 70 mg/kg b.w. i.p. lithium, and 5 mg/kg b.w. i.p. lamotrigine, respectively. There were 6 rats housed in each cage and weights of 1% saccharine solution and water bottles were measured at 2 h, following drug dosing, for each cage, for 4 days.
Rats were administered 10 mg/kg b.w. subcutaneous (s.c.) morphine for 7 days for sensitization as described earlier.2223 The locomotor activity of the rats was evaluated in terms of the frequency of crossings and rearing of the rats by placing them in a box sized 30×30×30 cm, divided in 9 squares, by vertical and horizontal lines separated by 10 cm each. A crossing was defined as movement of the rat from one square to another. Rearing was defined as standing of the rat on its 2 hind limbs. Sensitized rats with a 25% increase in baseline crossing or rearing activity (our unpublished data) were screened and were randomly divided into 3 groups (n=6) and were treated with 0.5 mL i.p. normal saline, 70 mg/kg b.w. i.p. lithium, and 5 mg/kg b.w. i.p. lamotrigine, respectively. Post-treatment locomotor activity was recorded, in terms of the number of crossings and rearing of the rats in 30 min, in all the 3 groups, 5 min after drug injection.
A statistically significant increase in the immobility time was observed in the lithium group in the TST, but not in the FST, compared to the control group. In the CST, the lithium group showed a maximal increase of 1% sucrose solution consumption at the 3rd week (p=0.0001) of treatment. There was no significant difference found between the 1% sucrose consumption of the control group and the lithium group of the non-stressed rats (Table 1).
In the isolation-induced aggression model, lithium-treated rats showed a statistical significant decrease in aggressive behaviour i.e. approach (p=0.0001), attack (p=0.006), bite (p=0.004), compared to the control group. However, no significant difference was found in the latency time of the first attack. The saccharine preference test did not reveal any significant decrease in the consumption of saccharine by the lithium-treated rats throughout the duration of 4 days. The morphine-sensitized hyperlocomotion model showed that rats treated with lithium showed a significant reduction in the crossing parameter (p=0.04) but not in the rearing parameter (Table 1).
A significant increase in the immobility time was observed in both the FST and the TST (p<0.05 and p<0.01, respectively), in the lamotrigine-treated rats, compared to the control group. A maximum increase of 1% sucrose consumption was observed at the 3rd week of treatment with lamotrigine (p=0.0001), in the CST. Lamotrigine did not change the sucrose consumption in the non-stressed rats (Table 1).
Lamotrigine markedly reduced the aggressive behaviour i.e. approach (p=0.0001), attack (p=0.002), and the bite parameter showed a trend towards significance (p=0.051), compared to the control group, in the isolation-induced aggression model. There was no significant difference found in the 1% saccharine solution consumption with lamotrigine treatment, in the saccharine solution preference test. In the morphine-sensitized hyperlocomotion model, lamotrigine-treated rats did not show any significant difference in the crossing and rearing parameters, compared to the control group (Table 1).
The present pharmacotherapy for BD is classified as lithium, anticonvulsants, and atypical antipsychotics.24 The discovery of the efficacy of lithium in BD was a serendipitous event. The development of anticonvulsants as carbamazepine, valproate, lamotrigine, etc. in BD was based on the hypothesis that epilepsy and BD share similar features such as kindling. The atypical antipsychotics approved for BD were developed on the buttress that typical antipsychotics as chlorpromazine tranquilized agitated/manic patients.14 The relatively unfolded etiopathogenesis of BD, the cyclic nature of the disease, and the absence of 'affect' in animals have made it extremely difficult for behavioural researchers to create new animal models.5613
The efficacy of the 2 therapeutic agents utilized in the present study, lithium and lamotrigine, is well established in BD. Lithium acts in BD by suppressing the inositol signalling through depletion of intracellular inositol and by inhibiting glycogen synthase kinase-3 (GSK-3).2 Lamotrigine preferentially inhibits neuronal hyperexcitability and modifies synaptic plasticity via inhibition of neuronal voltage-activated Na+ channels and possibly high-voltage-activated Ca2+ channels subsequently decreasing excessive transmitter release in the brain.25 The dose, duration, and schedule of administration of lithium and lamotrigine were extrapolated from previously published animal studies.891011
Depression and mania are the 2 most important facets of BD, hence animal models targeting these two facets were chosen in the present study. The animal models employed to evaluate the depression facet of BD were FST, TST, and CST. FST and TST have been thought to create behavioural despair in rats so that they lose hope to escape the stressful environment. Lack of escape-related behaviour in the rats is considered immobility.26 The CST is also one of the models employed to screen antidepressants. It is believed that the chronic mild stress induced in rats is analogous to the psychological stress that humans face, implicated in the aetiology of depression. The increase in the sucrose-seeking behaviour in chronically stressed rats after drug therapy is thought to be representing a reward-seeking behaviour, which is usually absent in anhedonia.27 Certainly each of these 3 models do not represent the entire spectrum of the depression facet of BD and it is unclear to what extent the underlying neurobiological/etiological changes produced in the animals overlap with those observed in humans. However, these limitations should not devalue their substantial predictive and face validities.
In the present study, the CST showed maximum predictive validity amongst models targeting the depression facet of BD as both lithium and lamotrigine showed statistically superior results compared to the FST and TST. This finding is seconded by a previous study which showed that lithium potentiated the antidepressant effect of imipramine and fluoxetine in rats exposed to chronic mild stress.28 Also, a previous study demonstrated that a 4 week dosing of lamotrigine exhibited antidepressant activity in the CST by reversal of the lipid peroxide levels.29 The lamotrigine-induced decrease in immobility time in the FST and TST is consistent with previous findings.1030 In the present study, lithium did not show any significant change in the immobility time in the FST. The reason for this observation could have been the low dose and duration of lithium therapy (70 mg/kg b.w. i.p. per day). An earlier study had demonstrated that a higher dose of lithium (4 g/kg b.w. i.p.) for 10 days produced a significant decrease in the immobility time in the FST.9 Also, lithium chow administered for 10-28 days decreased the immobility time significantly.31
The animal models employed to evaluate the mania facet of BD were the isolation-induced aggression test, saccharine preference test, and morphine-sensitized hyperlocomotion test. Isolation results in formation of an aggressive behaviour pattern usually absent in normally reared rats. Increase in the brain level of serotonin, dopamine, and norepinephrine has been associated with this increased aggression.19 Rats with a high preference for saccharine may represent the reward-seeking behaviour observed in mania, hence can be incorporated in a model to screen mood stabilizers. This model is based on the corollary that rats with a low preference for saccharine are usually associated with higher anxiety or have been exposed to depressogenic manipulations.21 Repeated morphine treatment in rodents shows a sensitized response i.e., an increase in the locomotor activity, rearing, and stereotypy movements. This drug-induced increased locomotor activity is linked with increased dopamine biosynthesis and dopamine-1 receptor firing in the mesolimbic area of the brain.22 Each of these 3 models employed in the present study have substantial amount of face and predictive validities and address aggression, reward-seeking, and hyperactivity, respectively, which are features of the mania phase of BD.
In the present study, amongst the 3 models employed to evaluate mania, the isolation-induced aggression model was found to have the highest predictive validity with superior significance for both lithium and lamotrigine. The significant decrease in the aggressive behaviour in the lithium and lamotrigine groups is in accordance with previous studies.811 The lack of significant reduction in the morphine-sensitized hyperlocomotion in the lamotrigine-treated rats could be due to the short duration of the dosing or due to the difference in the strain of rats employed in the present study, hence future studies are needed to establish the dose-response and dosing regimen for lamotrigine. The negligible change in the saccharine preference in both the lithium and lamotrigine-treated rats also may be due to strain/specie difference of the rodents employed in the present study. The Black Swiss strain of mice is identified as having high baseline saccharine consumption, hence is usually preferred in this test.32 A previous study has reported a failure for saccharine preference in the Wistar and Sprague-Dawley strains of rats despite exposure to chronic mild stress.33 Further studies are required to study the effect of chronic and multiple doses of lithium and lamotrigine in Wistar rats.
From the above observations the authors propose that CST and isolation-induced aggression test should be a part of a battery of tests used to evaluate mood stabilizers rather than an independent model due to the numerous limitations of present study. Notably, genetically manipulated models and kindling models were not employed in the study. The predictive validities of other therapeutic agents as valproate, carbamazepine, and atypical antipsychotics were also not evaluated. Amphetamine-induced hyperlocomotion model, considered as the gold standard model for mania was not included in the study due to significant procedural hurdles in its procurement even for scientific research. Further studies utilizing the above mentioned models along with other standard drugs would help create a comprehensive battery of tests, addressing various facets of BD, to evaluate mood stabilizers and help congregate the data obtained from the present study.
In conclusion, the CST and isolation-induced aggression test have the highest predictive validity amongst the models of depression/mania evaluated in the present study, which is a step forward to create a battery of tests for screening novel agents useful in BD, wherein each test is partially valid but the combined battery has a stronger power and validity.
Acknowledgments
The authors are thankful to Mr. Dharmender and Mr. Anil Kumar for providing a helping hand in taking care of the rats in the Animal House. Torrent Pharmaceuticals Limited, New Delhi is acknowledged for providing the authors with lithium and lamotrigine salts, free of charge.
References
1. Chiu JF, Chokka PR. Prevalence of Bipolar Disorder symptoms in Primary Care (ProBiD-PC): A Canadian study. Can Fam Physician 2011;57:e58-e67. PMID: 21642707.


2. Meltzer H. Basic and Clinical Pharmacology. In: Katzung BG, Masters SB, Trevor AJ, editor. Antipsychotic Agents and Lithium. 12th Ed. New York: McGraw-Hill, 2011, p. 517-1245.
3. Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account sub- threshold cases. J Affect Disord 2003;73:123-131. PMID: 12507745.


4. Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA 2005;293:2528-2530. PMID: 15914754.


5. Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neurosci Biobehav Rev 2007;31:825-831. PMID: 17628675.



6. Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci 2010;13:1161-1169. PMID: 20877280.



7. Einat H. Establishment of a battery of simple models for facets of bipolar disorder: a practical approach to achieve increased validity, better screening and possible insights into endophenotypes of disease. Behav Genet 2007;37:244-255. PMID: 16865528.


8. Gambarana C, Mangiavacchi S, Masi F, Scheggi S, Tagliamonte A, Tolu P, et al. Long-term lithium administration abolishes the resistance to stress in rats sensitized to morphine. Brain Res 2000;877:218-225. PMID: 10986335.


9. Gould TD, O'Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology 2008;54:577-587. PMID: 18096191.


10. Consoni FT, Vital MA, Andreatini R. Dual monoamine modulation for the antidepressant-like effect of lamotrigine in the modified forced swimming test. Eur Neuropsychopharmacol 2006;16:451-458. PMID: 16503122.


11. Arban R, Maraia G, Brackenborough K, Winyard L, Wilson A, Gerrard P, et al. Evaluation of the effects of lamotrigine, valproate and carbamazepine in a rodent model of mania. Behav Brain Res 2005;158:123-132. PMID: 15680200.


12. Kato T, Kubota M, Kasahara T. Animal models of bipolar disorder. Neurosci Biobehav Rev 2007;31:832-842. PMID: 17466374.


13. Markou A, Chimmulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology 2009;34:74-89. PMID: 18830240.


14. Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol 2011;164:1263-1284. PMID: 21410454.



15. Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther 1997;282:967-976. PMID: 9262365.

16. Chermat R, Thierry B, Mico JA, Steru L, Simon P. Adaptation of the tail suspension test to the rat. J Pharmacol 1986;17:348-350. PMID: 3795979.

17. Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 1992;16:525-534. PMID: 1480349.


18. Sánchez C, Gruca P, Papp M. R-citalopram counteracts the antidepressant-like effect of escitalopram in a rat chronic mild stress model. Behav Pharmacol 2003;14:465-470. PMID: 14501259.

19. Navarro JF, Manzaneque JM. Acute and subchronic effects of tiapride on isolation-induced aggression in male mice. Pharmacol Biochem Behav 1997;58:255-259. PMID: 9264100.


20. Tiwari OP, Bhattamisra SK, Tripathi PK, Singh PN. Anti-aggressive activity of a standardized extract of Marsilea minuta Linn in rodent models of aggression. Biosci Trends 2010;4:190-194. PMID: 20811139.

21. Flaisher-Grinberg S, Overgaard S, Einat H. Attenuation of high sweet solution preference by mood stabilizers: a possible mouse model for the increased reward-seeking domain of mania. J Neurosci Methods 2009;177:44-50. PMID: 18930764.


22. Grappi S, Marchese G, Secci ME, De Montis MG, Gambarana C, Scheggi S. Morphine sensitization as a model of mania: comparative study of the effects of repeated lithium or carbamazepine administration. Pharmacol Biochem Behav 2011;99:749-758. PMID: 21741986.


23. Hodgson SR, Hofford RS, Roberts KW, Wellman PJ, Eitan S. Socially induced morphine pseudosensitization in adolescent mice. Behav Pharmacol 2010;21:112-120. PMID: 20215964.

24. Abulseoud OA, Camsari MU, Ruby CL, Mohamed K, Gawad NM, Kasasbeh A, et al. Lateral hypothalamic kindling induces manic-like behavior in rats: a novel animal model. Int J Bipolar Disord 2014;2:7PMID: 26092394.



25. Ketter TA, Manji HK, Post RM. Potential mechanisms of action of lamotrigine in the treatment of bipolar disorders. J Clin Psychopharmacol 2003;23:484-495. PMID: 14520126.


26. Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 2011;Chapter 8:Unit 8.10APMID: 21462162.

27. Willner P. Validity, reliability and utility of the chronic mild stress (CMS) model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319-329. PMID: 9452163.

28. Sluzewska A, Szczawinska K. Lithium potentiation of antidepressants in chronic mild stress model of depression in rats. Behav Pharmacol 1996;7(Suppl 1):105PMID: 11224401.


29. Eren I, Naziroğlu M, Demirdaş A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem Res 2007;32:1188-1195. PMID: 17401662.


30. Bourin M, Masse F, Hascoët M. Evidence for the activity of lamotrigine at 5-HT (1A) receptors in the mouse forced swimming test. J Psychiatry Neurosci 2005;30:275-282. PMID: 16049571.


31. Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology 2007;32:2173-2183. PMID: 17299510.


32. Einat H. Different behaviors and different strains: potential new ways to model bipolar disorder. Neurosci Biobehav Rev 2007;31:850-857. PMID: 17307253.


33. Harris RB, Zhou J, Youngblood BD, Smagin GN, Ryan DH. Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav 1997;63:91-100. PMID: 9402621.