|
 |
- Search
| Psychiatry Investig > Volume 14(1); 2017 > Article |
Abstract
Objective
This retrospective case series study of the effectiveness of electroconvulsive therapy (ECT) augmentation on clozapine-resistant schizophrenia was conducted by EMR review.
Methods
Clozapine-resistance was defined as persistent psychotic symptoms despite at least 12 weeks of clozapine administration with blood levels over 350 ng/mL in order to rule out pseudo-resistance. Seven in-patients who were taking clozapine and treated with ECT were selected. We analyzed the psychopathology and subscales changed by ECT.
Results
The average number of ECT sessions was 13.4 (┬▒4.6). Total Positive and Negative Syndrome Scale (PANSS) score was significantly reduced by 17.9 (┬▒12.8) points (p=0.0384) on average, which represented a reduction of 25.5% (┬▒14.3). 71.4% (5/7) of patients were identified as clinical remission, with at least a 20% reduction in PANSS score. PANSS reduction was associated with number of ECT sessions, stimulus level in the final session, and blood clozapine levels before ECT. However, the negative subscale on the PANSS were not reduced by ECT in any patient. We did not observe any persistent adverse cognitive effects.
Though clozapine is the gold standard for the treatment of patients with antipsychotic-resistant schizophrenia, who represent as many as 20% to 30% of patients with schizophrenia,123 clinical symptoms persist in approximately 40 to 70% of clozapine users even after one year of medication.45 For clozapine-resistant schizophrenia, a variety of pharmacological and non-pharmacological approaches, including electroconvulsive therapy (ECT), have been tried as adjunct therapies.6 The reported advantages of various psychotropic drugs that is the most frequently attempted strategy have been known as modest or equivocal.56789 ECT augmentation has been continued ever since the re-introduction of clozapine in the 1990s10 and the effectiveness of ECT in patients on clozapine has been reported in two randomized controlled studies,411 as well as in case reports and open trials.51213141516171819 These studies consistently demonstrated favorable clinical effects and safety. In studies including a meta-analysis, 47.4-72.7% of patients with clozapine-resistant schizophrenia experienced clinical improvement after ECT.4202122 Further studies are required to validate the usefulness of ECT in clozapine-resistant schizophrenia.6
Clozapine resistance is defined as the persistence of psychotic symptoms even after at least 12 weeks of clozapine administration and despite a clozapine blood level of more than 350 ng/mL.45 Before examining the effects of ECT augmentation on clozapine resistance, pseudo-resistance arising from drug noncompliance, the effects of other medications, misdiagnosis, and adverse events must be ruled out.1 However, most studies did not provide information on dosages and blood levels of clozapine, which would be necessary to confirm the effects of ECT augmentation.2324 In order to clarify the effects of ECT augmentation on clozapine-resistant schizophrenia, we conducted a case series study that included only patients whose blood clozapine levels were maintained above 350 ng/mL for a sufficient period of time,4525 and analyzed the psychopathology and symptom domains changed by ECT procedures.
The electronic medical record (EMR) of Dongguk University International Hospital was retrospectively reviewed to identify patients diagnosed with schizophrenia by DSM-IV-TR criteria who were treated with clozapine and ECT from December 2012 to April 2015. Patients whose psychotic symptoms were persistent or only partially responsive to 12 weeks or more of clozapine administration and who consistently had blood levels of clozapine greater than 350 ng/mL were eligible for the study. Patients with physical illnesses, substance addiction, or IQs less than 80 were excluded.
The demographic characteristics, psychiatric history including clozapine administration, and clinical and laboratory findings of patients before and after ECT were collected. Psychotic symptoms were evaluated using the Positive and Negative Syndrome Scale (PANSS), in which each item was assessed on a score of 0-6 because the responses could possibly be underestimated when using a score of 1-7.26 A lower PANSS score indicates fewer symptoms. Changes in PANSS score following ECT are given as mean scores and percentage change. The symptom domains were also explored with Hwang's five factors model, which was derived from the Korean version of the PANSS; the symptom domains are positive, negative, activation, autistic preoccupation, and anxiety/depressive factors.27
Adverse events after ECT were tallied using progress notes and the cognitive effects of ECT were assessed before and after the last ECT session using the Mini-Mental State Examination, Korean version of the Consortium (MMSE-KC).28 This study was approved by the Institutional Review Board of Dongguk University International Hospital.
ECT was performed three times per week using bilateral electrode placement and brief pulse stimuli (800 mA; 1 ms) with a MECTA spECTrum 5000Q (MECTA Corp, Lake Oswego, OR, USA). ECT was begun at level 3 (80 millicouloumbs; mC) for men and at level 2 (42 mC) for women, according to Coffey et al.29 Glycopyrrolate (0.1-0.2 mg/Kg) was administered intravenously a few minutes before the patients were given anesthesia for ECT. Thiopental (2-4 mg/kg) was most often used for the general anesthesia. Succinylcholine (0.5-1 mg/kg) was injected to produce muscular relaxation, which was confirmed by fasciculation. Seizure was followed up with EEG, ECG, and visual observation.
Nominal data, such as total and symptom domain PANSS scores, were analyzed with paired t-tests. Linear regression was used to explore the factors on the PANSS that changed after ECT. Statistical significance was set at p<0.05. All statistical analyses were performed using SPSS ver. 21.0 for Windows (SPSS, Inc., Chicago, IL, USA).
The mean age and length of education of the seven patients were 41.4 (┬▒14.2) and 13.7 (┬▒2.1) years, respectively. The mean durations of illness and clozapine administration were 15.0 (┬▒8.6) and 3.9 (┬▒13.4) years, respectively (Table 1).
The mean dosages of clozapine taken by the seven patients before and after ECT were 350.0 (┬▒146.5) and 260.7 (┬▒95.6) mg, respectively (p=0.048; t=2.471, df=6). The main reason for the reduction in mean dose was that one patient showed transient postictal delirious episodes late in the course of ECT, so her dosage was reduced. There were no statistical differences in clozapine dose during the course of ECT in the other six patients. The mean blood levels of clozapine and norclozapine were 637.9 (┬▒141.3) ng/mL and 275.5 (┬▒72.1) ng/mL before ECT and 574.7 (┬▒244.5) ng/mL and 293.5 (┬▒180.9) ng/mL after ECT, respectively. There were no statistical differences in blood levels over the ECT course (Table 2).
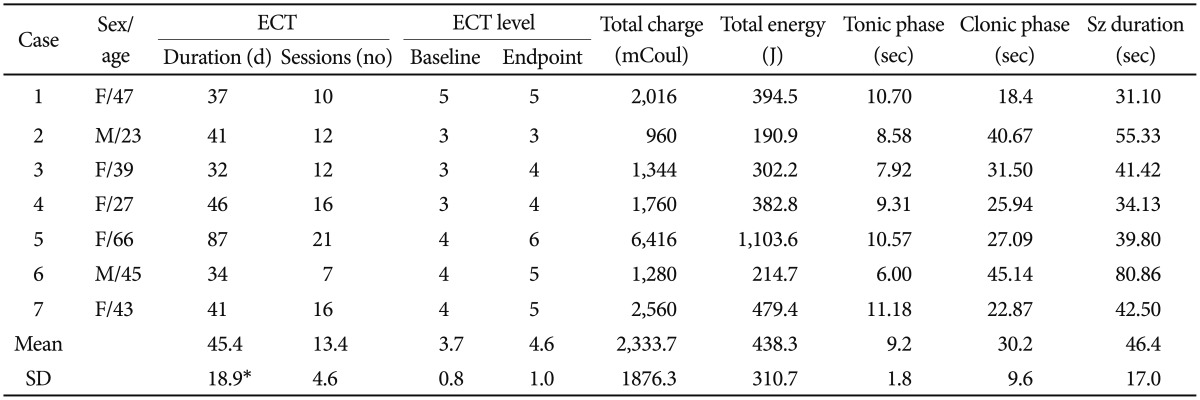
The main indication for ECT augmentation was insufficient clinical response to clozapine. The mean number of sessions and duration of ECT were 13.4 (┬▒4.6) and 45.4 (┬▒18.9) days, respectively (Table 3). The mean total PANSS scores before and after ECT were 70.1 (┬▒17.9) and 52.3 (┬▒17.9) points, respectively, using the 0-6 scoring system, which is a reduction in mean score of 17.9 (┬▒12.8) points and a statistically significant decrease (p=0.0384). The mean percentage score reduction was 25.5% (┬▒14.3). 71.4% (5/7) of patients were identified as being in clinical remission, as defined by a 20% reduction in PANSS score. In the other two patients, one experienced a reduction in symptoms of 19.3%, and the other experienced no change at all (case 2). In the linear regression analysis, the factors that were associated with a decrease in PANSS score were total number of ECT sessions (╬▓=-0.722), stimulus level in the final session (╬▓=1.182), and blood clozapine level before ECT (╬▓=-0.600). In Hwang's five factor model of the PANSS, the positive factor (p=0.0090; t=3.7932, df=6), the activation factor (p=0.0139; t=3.4340, df=6), autistic preoccupation (p=0.0109; t=3.6332, df=6), and anxiety/depression (p=0.0437; t=2.5460, df=6) were reduced significantly, while the negative factor was not affected. No patient showed any persistent adverse cognitive effects as assessed by the MMSE-KC after ECT.
During the course of ECT, six of the seven patients were maintained on clozapine with no significant changes in dose, and their blood clozapine levels were consistently over 350 ng/mL. One patient (case 5) showed post-ECT irritability manifesting as transient disorientation of time and a mild delirious state, which seemed to occur at the high electrical dose, level 7 (432 mC) and was relieved by benzodiazepines. Reducing her dose of clozapine and the electrical stimulus level, and providing hyperventilation for a few minutes produced successful seizures without causing delirious events afterwards. Despite the difficulties she had, she responded well to ECT and experienced a reduction in PANSS score of 28.1%. Four patients (cases 1, 3, 4, and 6) complained of mild headaches and temporary disorientations of time which were easily relieved with painkillers and bed rest. Only one patient (case 2) did not respond at all to 12 sessions of ECT and had no change in PANSS score. His PANSS profile was the negative type put forward by Kay et al.,30 and his negative scale score was not changed by ECT. The total score on the PANSS is a trade-off between the positive and general psychopathology scales. The other six patients were all positive type.
This study is a case series report using a retrospective EMR review that aimed to explore the efficacy and safety of ECT in patients with clozapine-resistant schizophrenia. We first ruled out the possibility of pseudo-resistance1 by ensuring that blood clozapine levels of patients were maintained above 350 ng/mL for a sufficient period of time.45 On average, the seven patients experienced clinical benefits from ECT, with a mean reduction of 25.5% on the PANSS, without serious adverse events. The clinical remission rate was 71.4%, which corresponds with the results of previous studies. Kho et al.18 reported a 30% reduction in PANSS score in 8 (72.7%) out of 11 patients with clozapine-resistant schizophrenia treated with ECT, and Petrides et al.4 showed a 20% reduction in PANSS score in 12 (60%) out of 20 patients. The present study provides further evidence for the benefits of ECT augmentation on patients with clozapine-resistant schizophrenia and shows that ECT causes no persistent adverse cognitive events,1 even though the mean dosages and blood levels of clozapine were not substantially changed during the course of ECT. Reductions in PANSS score were associated with total number of ECT sessions, the last stimulus level, and blood clozapine levels before ECT.
In this report, the negative subscale on the PANSS by Kay et al.,30 was not significantly reduced by ECT; one patient (case 2), who did not respond to ECT at all, was classified as the negative type. The average score on the negative subscale in all seven patients was not statistically reduced (data not shown). This is in agreement with other studies that showed that patients who responded to ECT were less likely to be negative type,313233 and that there is less of a reduction in the negative subscale compared with the positive and general subscales following ECT.34 However, the seven patients in this study had chronic schizophrenia and their psychotic symptoms were resistant to various antipsychotic medications, including clozapine, which they had taken for 3.9 (┬▒3.4) years on average. It has been suggested that long-term treatment may be required to observe improvements in negative symptoms.35 Therefore, this study may have been insufficiently long to evaluate improvements in negative symptoms following a course of ECT as the average course of ECT took place over only 45.4 (┬▒18.9) days.
Some limitations to this study should be noted. First, this is a case series report with only seven patients, so the findings are not necessarily generalizable, although the findings of this study do avoid the crucial drawback, common to other studies, of patients possibly being underdosed with clozapine.6 A larger number of cases and studies with more participants are needed to validate the study findings. Second, this study also did not have controls such as sham ECT or combinations of other pharmacotherapies. Third, nearly half (3/7) of the patients were taking other antipsychotic drugs along with clozapine, which may have afffected the usefulness of clozapine. However, chlorpromazine-equivalent dosages were not statistically different before and after ECT. We will attempt to rule out the effects of other antipsychotic drugs in our next study. Fourth, this report did not trace clinical courses of patients for a long period of time after ECT. Further studies will be required to determine whether the effects of ECT augmentation will persist and prevent the recurrence of psychotic symptoms. Even though this was a retrospective study conducted by reviewing the EMR, our results strongly suggest that ECT augmentation could be a favorable strategy for the treatment of clozapine-resistant schizophrenia.
Acknowledgments
This research was supported by Free Research Project (S-2015-G0041-00058), Dongguk University Medical Center and the Institute of Clinical Psychopharmacology, Dongguk University College of Medicine.
References
1. Dold M, Leucht S. Pharmacotherapy of treatment-resistant schizophrenia: a clinical perspective. Evid Based Ment Health 2014;17:33-37. PMID: 24713315.


2. Juarez-Reyes MG, Shumway M, Battle C, Bacchetti P, Hansen MS, Hargreaves WA. Effects of stringent criteria on eligibility for clozapine among public mental health clients. Psychiatr Serv 1995;46:801-806. PMID: 7583481.


3. Meltzer HY. Treatment-resistant schizophrenia--the role of clozapine. Curr Med Res Opin 1997;14:1-20. PMID: 9524789.


4. Petrides G, Malur C, Braga RJ, Bailine SH, Schooler NR, Malhotra AK, et al. Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: a prospective, randomized study. Am J Psychiatry 2015;172:52-58. PMID: 25157964.


5. Remington G, Saha A, Chong SA, Shammi C. Augmentation strategies in clozapine-resistant schizophrenia. CNS Drugs 2005;19:843-872. PMID: 16185094.


6. Miyamoto S, Jarskog LF, Fleischhacker WW. New therapeutic approaches for treatment-resistant schizophrenia: a look to the future. J Psychiatr Res 2014;58:1-6. PMID: 25070124.


7. Kerwin RW, Bolonna A. Management of clozapine-resistant schizophrenia. Adv Psychiatr Treat 2005;11:101-106.

8. Shiloh R, Zemishlany Z, Aizenberg D, Radwan M, Schwartz B, Dorfman-Etrog P, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry 1997;171:569-573. PMID: 9519099.


9. Kerwin R. From pharmacological profiles to clinical outcomes. Int Clin Psychopharmacol 2000;15(Suppl 4):S1-S4.
10. Fink M. Clozapine and electroconvulsive therapy. Arch Gen Psychiatry 1990;47:290-291. PMID: 1968331.


11. Masoudzadeh A, Khalilian AR. Comparative study of clozapine, electroshock and the combination of ECT with clozapine in treatment-resistant schizophrenic patients. Pakistan Journal of Biological Sciences. Pak J Biol Sci 2007;10:4287-4290. PMID: 19086588.


12. Williams L, Newton G, Roberts K, Finlayson S, Brabbins C. Clozapine-resistant schizophrenia: a positive approach. Br J Psychiatry 2002;181:184-187. PMID: 12204919.


13. Friedel RO. The combined use of neuroleptics and ECT in drug resistant schizophrenic patients. Psychopharmacol Bull 1986;22:928-930. PMID: 3797594.

14. Safferman AZ, Munne R. Combining Clozapine with ECT. Convuls Ther 1992;8:141-143. PMID: 11941161.

15. Benatov R, Sirota P, Megged S. Neuroleptic-resistant schizophrenia treated with clozapine and ECT. Convuls Ther 1996;12:117-121. PMID: 8744173.

16. Bhatia SC, Bhatia SK, Gupta S. Concurrent administration of clozapine and ECT: a successful therapeutic strategy for a patient with treatment-resistant schizophrenia. J ECT 1998;14:280-283. PMID: 9871852.


17. Kales HC, Dequardo JR, Tandon R. Combined electroconvulsive therapy and clozapine in treatment-resistant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 1999;23:547-556. PMID: 10378236.


18. Kho KH, Blansjaar BA, de Vries S, Babuskova D, Zwinderman AH, Linszen DH. Electroconvulsive therapy for the treatment of clozapine nonresponders suffering from schizophrenia--an open label study. Eur Arch Psychiatry Clin Neurosci 2004;254:372-379. PMID: 15538604.


19. Grover S, Hazari N, Kate N. Combined use of clozapine and ECT: a review. Acta Neuropsychiatr 2015;27:131-142. PMID: 25697225.


20. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, Paplos KG, Soldatos CR. Concurrent administration of clozapine and electroconvulsive therapy in clozapine-resistant schizophrenia. Clin Neuropharmacol 2006;29:52-56. PMID: 16518135.


21. Kupchik M, Spivak B, Mester R, Reznik I, Gonen N, Weizman A, et al. Combined electroconvulsive-clozapine therapy. Clin Neuropharmacol 2000;23:14-16. PMID: 10682225.


22. Lally J, Tully J, Robertson D, Stubbs B, Gaughran F, MacCabe JH. Augmentation of clozapine with electroconvulsive therapy in treatment resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res 2016;171:215-224. PMID: 26827129.


23. Porcelli S, Balzarro B, Serretti A. Clozapine resistance: augmentation strategies. Eur Neuropsychopharmacol 2012;22:165-182. PMID: 21906915.


24. Muscatello MR, Bruno A, De Fazio P, Segura-Garcia C, Pandolfo G, Zoccali R. Augmentation strategies in partial responder and/or treatment-resistant schizophrenia patients treated with clozapine. Expert Opin Pharmacother 2014;15:2329-2345. PMID: 25284216.


25. Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull 2010;36:71-93. PMID: 19955390.


26. Leucht S, Kissling W, Davis JM. The PANSS should be rescaled. Schizophr Bull 2010;36:461-462. PMID: 20357133.



27. Hwang SS, Chang JS, Lee KY, Kim SH, Ahn YM, Kim YS. Causal model of insight and psychopathology based on the PANSS factors: 1-year cross-sectional and longitudinal revalidation. Int Clin Psychopharmacol 2009;24:189-198. PMID: 19521247.


28. Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci 2002;57:P47-P53. PMID: 11773223.


29. Coffey CE, Lucke J, Weiner RD, Krystal AD, Aque M. Seizure threshold in electroconvulsive therapy: I. Initial seizure threshold. Biol Psychiatry 1995;37:713-720. PMID: 7640326.


30. Kay SR, Flszbein A, Opfer LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-276. PMID: 3616518.


31. Chanpattana W, Chakrabhand MLS, Kongsakon R, Techakasem P, Buppanharun W. Short-term effect of combined ECT and neuroleptic therapy in treatment-resistant schizophrenia. J ECT 1999;15:129-139. PMID: 10378152.


32. Chanpattana W, Chakrabhand ML. Combined ECT and neuroleptic therapy in treatment: prediction of outcome. Psychiatry Res 2001;105:107-115. PMID: 11740980.


33. Payne NA, Prudic J. Electroconvulsive therapy: Part I. A perspective on the evolution and current practice of ECT. J Psychiatr Pract 2009;15:346-368. PMID: 19820553.



34. Pawelczyk T, Kołodziej-Kowalska E, Pawełczyk A, Rabe-Jabłon´ska J. Augmentation of antipsychotics with electroconvulsive therapy in treatment-resistant schizophrenia patients with dominant negative symptoms: a pilot study of effectiveness. Neuropsychobiology 2014;70:158-164. PMID: 25358377.


35. Schooler NR, Buchanan RW, Laughren T, Leucht S, Nasrallah HA, Potkin SG, et al. Defining therapeutic benefit for people with schizophrenia: focus on negative symptoms. Schizophr Res 2015;162:169-174. PMID: 25579053.