INTRODUCTION
Bobble-head doll syndrome (BHDS) is a rare neurological stereotypic head movement disorder characterized by about 2 Hz to 3 Hz of periodic anterior-posterior and occasionally side-to-side head movements [1,2]. Organically, this syndrome is almost always associated with a cystic lesion in or near the third ventricle, causing pressure on periventricular neuronal structures, including the thalami. Previously reported causes include suprasellar arachnoid cyst (AC); third ventricular cyst; cystic choroid plexus papilloma of the third ventricle; cyst in the cavum septum pellucidum; and less commonly, aqueductal stenosis, shunt malfunction, or Dandy-Walker complex malformation [1,3,4]. The rhythmic head bobbling is aggravated by emotional stress and can be decreased in frequency voluntarily and stops during sleep. The syndrome commonly presents prior to 10 years of age, and surgical treatment is the only treatment method, including cyst decompression by microsurgical fenestration of the cyst via a transcranial approach or by insertion of a device such as a cysto-peritoneal shunt [1,2,5].
Here, we report a case of BHDS with hypomania, rarely combined symptoms caused by a large suprasellar AC. We share the experience of surgically treating the patientŌĆÖs stereotypy and mood disorder.
Case
A 22-year-old previously healthy man visited the outpatient department of neuropsychiatry in our institution. He had been suffering from involuntary abnormal head movement for 5 months. The movement was characterized by 4-5 Hz of anterior-posterior head movements, followed by 3-4 Hz of side-to-side movements. This head movement pattern was rhythmic, similar to that seen with a nodding- and shakinghead doll, and worsened under stressful situations. It could, however, be decreased voluntarily, and it disappeared completely during sleep.
He was first diagnosed at a local neuropsychiatric clinic as having a tic disorder and managed for the same; imaging was not performed because the abnormal head movement was stereotypic, as seen in tic disorder, obsessive-compulsive disorder, repetitive behavior, and other stereotypies. However, the movement gradually worsened, and even new psychiatric symptoms developed 3 months prior to presentation. The psychiatric symptoms included elated mood, talkativeness, inflated self-esteem, distractibility, and intermittent aggressiveness; these could be clinically diagnosed as hypomania. Initially, hypomania was not clinically significant, and only an observation was made. Finally, however, he was referred to our institution for further management because all symptoms had aggravated over time.
During baseline evaluation after admission, brain magnetic resonance imaging (MRI) revealed a large cystic lesion in the suprasellar region extending into the third ventricle, and hydrocephalus was observed in lateral ventricles (Figure 1). There was no enhancement or solid portion in the thin cyst wall, and the internal signal exhibited by the cystic contents was homogenous and suppressed in the fluid-attenuated inversion recovery sequence. Accordingly, a radiological diagnosis of suprasellar AC was made. The lateral ventricles were severely dilated, and the midbrain and pons were anteriorly compressed by the cyst, although the fourth ventricle was of normal size, indicating obstructive hydrocephalus. Although the optic chiasm, pituitary gland, and pituitary stalk were also severely compressed and displaced, visual disturbance was not observed, and the endocrine profile was normal. Combined with stereotypic head movement, similar to that in a bobble-head doll, these findings were indicative of bobble-head doll syndrome (BHDS).
Neurosurgeons performed endoscopic fenestration of the cyst wall to facilitate flow of cerebrospinal fluid (CSF) to the lateral ventricle and basal cistern. They performed a large fenestration to the lateral ventricle by incision of the cyst wall: an opening to the prepontine cistern by scissoring of the bottom of cyst wall and Liliequist membrane. Consequently, the cyst communicated with the lateral ventricle superiorly and with the prepontine cistern inferiorly. Subsequently, the cyst shrunk.
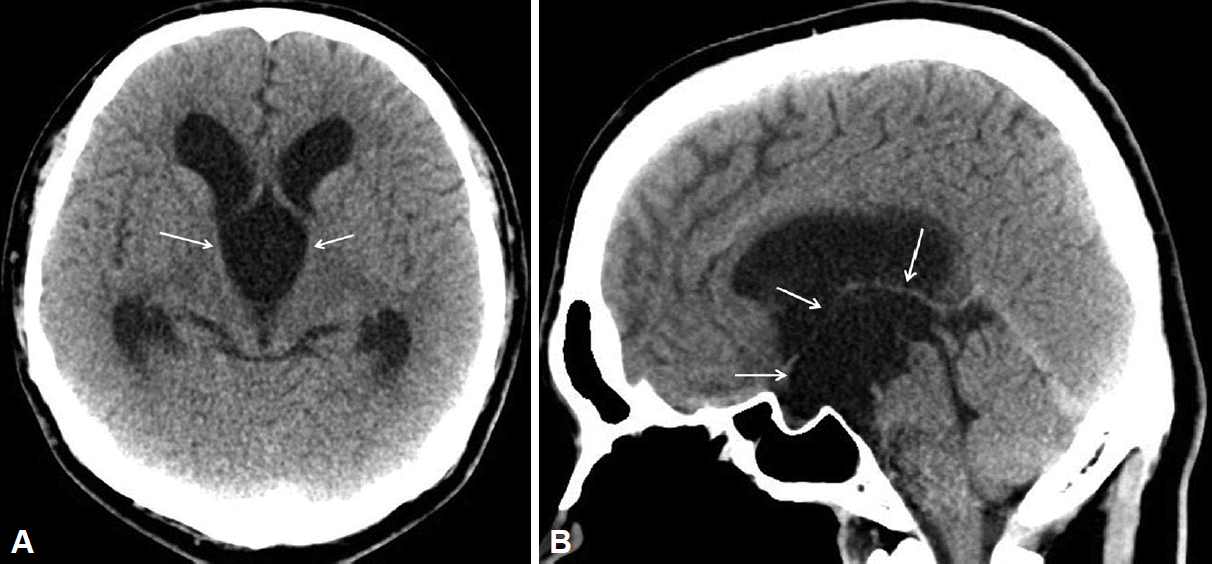
The patientŌĆÖs stereotypic head movement completely stopped on postoperative day 1. However, his hypomanic symptoms persisted, and 50 mg/day quetiapine, the atypical antipsychotic drug, was administered because he reported difficulty in controlling his behavior during hospitalization. After drug administration, he could maintain social relationships with surrounding people. Postoperative computed tomographic scanning performed 1 week later revealed a reduction in the size of the lateral ventricle and a decompressed cyst within the third ventricle (Figure 2). A month after surgery, other hypomanic symptoms also improved, and he was discharged with a euthymic status.
After discharge, the same dose of quetiapine was maintained for 2 months and then discontinued. During 4 years of follow-up observation without medication, neuropsychiatric symptoms including stereotypic head movement and hypomania did not relapse.
Discussion
Almost all patients with BHDS exhibit head movement in the vertical direction, similar to nodding. Unlike that in previous reports, our patient showed a mixed type of stereotypy: anterior-posterior followed by side-to-side head movements and accompanying mood symptoms. To our knowledge, this mixed pattern of BHDS with mood symptoms caused by a suprasellar AC has not previously been reported [6].
The pathophysiology of BHDS is not clearly understood. Benton et al. [7] suggested that in BHDS, distortion of the third ventricle by a cystic lesion causes impairment of normal function of the surrounding structures. Russo et al. [2] reported that the cause of BHDS was extrapyramidal dysfunction due to pressure from pulsation within the cyst on surrounding structures, such as the thalamus, basal ganglia, and motor cortex. In addition, they reported a pressure effect on the dorsomedial thalamic nucleus. Hagebeuk et al. [8] reviewed and discussed 30 cases of BHDS and suggested that the red nucleus was an important site of symptom onset and aggravation. Although many hypotheses have been proposed, the exact cause of BHDS remains unclear.
Suprasellar AC is one of common causes of BHDS and accounts for about 9% of intracranial ACs [9]. The majority of these lesions present as an asymptomatic mass; however, they can become symptomatic because of cyst enlargement or hemorrhage. With production of fluid from mesothelial cells of the cyst wall, the size of the AC might increase. Occasionally, one-way valve flow of CSF from subarachnoid space could increase the size of the cyst [10].
Accordingly, in our presented case, we hypothesized that the recent rapid growth in the pre-existing large suprasellar AC was because of the relatively late onset of symptoms, atypical mixed stereotypy pattern, and suspected imaging features. On brain MRI, some evidence of adaptive changes to long-standing hydrocephalus, such as the absence of effacement in the sulcal space and inapparent periventricular edema, was observed. Before the recent growth, the pressure effect of the AC on the periventricular neuronal structures was under the symptomatic threshold. Because of unknown provoking factors, the pressure effect of the AC abruptly increased beyond the threshold of adjacent neuronal structures, that is, presumably, the dorsomedial thalami, basal ganglia, red nucleus, and motor cortex. Head-bobbling then began.
In addition, we should focus on the manifestation of manic symptoms unique to this case. In a recently proposed consensus model for mania, ventral prefrontal-limbic modulation was found to play a key role in emotional function in humans [11-13]. In the context of the structural aspect, possible mechanisms of hypomanic symptoms due to the pressure effect of AC can be established. In imaging, direct pressure of the AC affected the bilateral thalami, globus pallidus, ventromedial striatum, and nucleus accumbens. Indirectly, secondary obstructive hydrocephalus transmits pressure to the corpus callosum, cingulate gyrus, and even the amygdala [13]. Pressure effects to this limbic region could produce a functional alteration, and mood symptoms could develop.
Mood symptoms are the result of complex modulation or regulation of neuronal networks [13]. Therefore, it probably takes time for structural influences to exert their functional influences. Moreover, mood symptoms are thought to be caused by wider structural influences. In this case, mood symptoms developed about 1-2 months after stereotypy began. After decompressive surgery of the AC, mood symptoms persisted for 1-2 months, and were managed with medication. This clinical course shows temporal correlation with onset features and supports our hypothesis. Our patient has been doing well for the past 4 years without recurrence of symptoms.
Conclusively, the long-standing hydrocephalus caused a subclinical extensive adaptive alteration in neural structures including the limbic system. Recent growth of the cyst may have triggered an unusually wide range of symptoms. We expect our case may provide some clues to structurally understand the pathology of bipolar disorder.
In summary, we report our successful experience of surgically treating stereotypy and hypomania developed in the rare BHDS. We suggest that there are surgically treatable stereotypies frequently encountered in clinical practice. Furthermore, if stereotypies do not respond to appropriate treatment and other symptoms are complicated, brain imaging might be essential.