Positive Association of TEAD1 With Schizophrenia in a Northeast Chinese Han Population
Article information
Abstract
Objective
Schizophrenia is a complex and devastating psychiatric disorder with a strong genetic background. However, much uncertainty still exists about the role of genetic susceptibility in the pathophysiology of schizophrenia. TEA domain transcription factor 1 (TEAD1) is a transcription factor associated with neurodevelopment and has modulating effects on various nervous system diseases. In the current study, we performed a case–control association study in a Northeast Chinese Han population to explore the characteristics of pathogenic TEAD1 polymorphisms and potential association with schizophrenia.
Methods
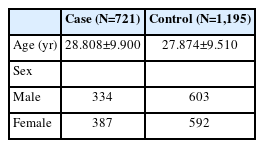
We recruited a total of 721 schizophrenia patients and 1,195 healthy controls in this study. The 9 single nucleotide polymorphisms (SNPs) in the gene region of TEAD1 were selected and genotyped.
Results
The genetic association analyses showed that five SNPs (rs12289262, rs6485989, rs4415740, rs7113256, and rs1866709) were significantly different between schizophrenia patients and healthy controls in allele or/and genotype frequencies. After Bonferroni correction, the association of three SNPs (rs4415740, rs7113256, and rs1866709) with schizophrenia were still evident. Haplotype analysis revealed that two strong linkage disequilibrium blocks (rs6485989-rs4415740-rs7113256 and rs16911710-rs12364619-rs1866709) were globally associated with schizophrenia. Four haplotypes (C-C-C and T-T-T, rs6485989-rs4415740-rs7113256; G-T-A and G-T-G, rs16911710-rs12364619-rs1866709) were significantly different between schizophrenia patients and healthy controls.
Conclusion
The current findings indicated that the human TEAD1 gene has a genetic association with schizophrenia in the Chinese Han population and may act as a susceptibility gene for schizophrenia.
INTRODUCTION
Schizophrenia is a common and severe mental illness with a lifetime risk of around 1% [1]. Although advances in genomics, epidemiology, and neuroscience have led to great progress in our understanding of schizophrenia over the past few decades, its etiology and pathophysiology remain unclear. Like other polygenic genetic diseases, the cause of schizophrenia is complex and multifactorial, with the contribution of many susceptibility genes, epigenetic and environmental factors, as well as random factors [2]. Studies of families, twins, and foster children have all shown that genetic factors are significant in the occurrence of schizophrenia, accounting for about 80% [3]. With the application of the genome-wide association study, more and more susceptible genes of small relation to the risk of schizophrenia have been discovered. Among them, a variety of genes are related to neural development. Neurodevelopmental hypothesis of schizophrenia is a broadly accepted paradigm based on epidemiology, anatomical, and neuroimaging studies, with the view that the symptoms of schizophrenia are the terminal result of developmental problems in the brain resulted from hereditary factors and perinatal environment factors.
TEA domain transcription factor 1 (TEAD1) is a member of the TEAD (TEA/ATTS domain) transcription factor family and mapped to the human chromosome 11p15, a potentially relevant region for schizophrenia [4,5]. TEAD proteins are evolutionarily conserved with an N terminal DNA-binding domain called the TEA domain and a C terminal protein-binding domain which co-activators bind to. TEAD1 can hardly induce transcription of target gene on its own [6]. As is known to all, TEAD1 is a key transcription factor in Hippo signaling pathway, a highly conserved pathway that plays a crucial role in neurodevelopmental stability and nervous system diseases controlled by modulating neuroinflammation, neuronal differentiation, and cell death [7]. Although the transcriptional activity of TEAD1 is traditionally believed to be regulated by the co-activators such as nuclear Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif, several studies suggested that TEAD1 is regulated through Hippo independent mechanisms [8]. Once TEAD1 binds its co-activators, the complexes can regulate the expression of target genes that play an important role in cell growth, proliferation, differentiation, apoptosis, and cell migration. Particularly, TEADs have been direct implicated in development, with expression detected in a wide range of embryonic brains as early as 2-cell stage, especially in forebrain [9]. During cortical development, the expression of TEAD1 increased significantly from the expansion and neurogenesis phase to the gliogenic phase, indicating potential specific functions [10]. TEADs affect the development of different tissues and organs, such as heart [11], muscle [12], neural crest and notochord [13,14], and trophectoderm [15], thus, dysfunction of TEADs can lead to various developmental disease. The knockout of TEAD1 has led to embryo death [11]. Overexpressing a transcriptionally active form of TEAD1 leads to severe dysplasia with reduced neuronal differentiation, increased neural progenitor cells (NPCs), the formation of tumor-like rosettes and injuries of ventricular surface [16]. In addition to development, TEAD1 also play a role in brain tumors generation [17].
In the present study, an independent case–control study was conducted to seek the genetic association of the human TEAD1 gene with schizophrenia in a Northeast Chinese Han population with 721 schizophrenia patients and 1,195 healthy controls.
METHODS
Subjects
We recruited 721 schizophrenia patients and 1,195 healthy controls (Table 1), all unrelated Han Chinese origin, from the Department of Psychiatry, Dalian Seventh People’s Hospital, Northeast China. Diagnoses were made strictly in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [18], based on the patients’ clinical symptoms, impaired social functioning, course of illness, by two senior psychiatrists. Healthy controls in this study were demographically matched to patients and all had been confirmed by a reliable psychiatrist that they were free of psychiatric disorders. Before any study-related procedures were performed, all participants were aware of the main purpose and methods of the study and provided written informed consent. The study was approved by the Ethics Committee of the Dalian Seventh People’s Hospital, China (ID: 2020-18).
Single nucleotide polymorphism selection
We downloaded information of all single nucleotide polymorphisms (SNPs) within and adjacent to the human TEAD1 gene from the International HapMap project database on db-SNP (http://www.ncbi.nlm.nih.gov/SNP/), and then selected potentially relevant 9 SNPs, of which the minor allele frequencies are all greater than 0.01. The 9 SNPs are evenly distributed in the TEAD1 gene region, and the distance between the adjacent two SNPs is less then 60 kb which is the predicted genetic linkage distance in human. In addition, the selected 9 SNPs covers about 255 kb in length, covering approximately 100% of the total length of the human TEAD1 gene, which is 270 kb.
Genotyping
The venous blood of all subjects was collected to extract genomic DNA by a commercially QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, German). Direct DNA sequencing or polymerase chain reaction (PCR)-restriction fragment length polymorphism analysis was used for genotyping of SNPs and Software Primer Premier 5.0 (Premier, Toronto, Canada) was used to design all PCR primer pairs. PCR products were either separated by agarose gel electrophoresis (2%–3% gel) stained with ethidium bromide after completely digested overnight with 4U of restriction enzyme or sequenced. All the results were independently read by two skilled technicians.
Statistics
All the data were analyzed by SHEsis software (SHEsis Online Version; http://analysis.bio-x.cn/SHEsisMain.htm) [19,20]. and Haploview (Broad Institute, Cambridge, MA, USA; https://www.broadinstitute.org/haploview/haploview). Chi-square test was applied to perform Hardy-Weinberg equilibrium on the whole sample. Statistical differences of allele and genotype frequencies between cases and controls were detected by Pearson χ2 test. D’ value was used to evaluate pairing linkage disequilibrium (LD) between alleles. Odds ratios and their 95% confidence interval were used to evaluate the statistical efficacy of different alleles and haplotypes. The Bonferroni correction was adopted to reduce the class I errors caused by multiple tests, that is, Bonferroni corrected p-value=α/the number of SNP sites. If the p-value of each SNP site is less than Bonferroni’s corrected p-value, it indicates that there is a significant correlation between this site and the disease. Criterion for statistical significance was set at two-tailed p<0.05.
RESULTS
Allele and genotype association of selected TEAD1 SNPs with schizophrenia
We have checked the expression profile of human TEAD1 gene in the human protein atlas database (https://www.proteinatlas.org/) [21,22], which indicated the TEAD1 gene is broadly expressed in brain tissue with low region specificity (Figure 1A and B). Data from Tabula Muris databases (https://tabulamuris.ds.czbiohub.org/) [23] show that TEAD1 mRNA is highly expressed in the Brain_Non-Myeloid, especially expressed in Bergmann glial cell, brain pericyte, and astrocyte of the cerebral cortex (Figure 1C). However, the data extracted from the SZDB database [24,25] showed that, other than other detected regions, a significant difference of TEAD1 expression in stratum existed between the schizophrenia patients and healthy control subjects (corrected p=0.001) (Figure 2). Therefore, we suspected that the differential expression of TEAD1 in striatum between schizophrenic patients and healthy controls indicates that the human TEAD1 gene may be associated with schizophrenia.

Expression profile of TEAD1 gene in human brain and specific mouse tissues. A: According to the human protein atlas database (https:// www.proteinatlas.org/), the human TEAD1 gene is broadly distributed in nearly all the human tissues including brain (https://www.proteinatlas. org/ENSG00000187079-TEAD1/tissue). B: The human TEAD1 gene is expressed widely in brain, and the gradient of the red color indicates the TEAD1 expression levels in different brain regions (https://www.proteinatlas.org/ENSG00000187079-TEAD1/brain). TPM on the vertical axis represents the transcript quantification value, and the horizontal axis represents different tissues. C: scRNA-seq analysis from Tabula Muris databases (https://tabula-muris.ds.czbiohub.org/) demonstrates that mouse TEAD1 mRNA is highly expressed in the Brain_Non-Myeloid, especially in Bergmann glial cell, brain pericyte and astrocyte. Adapted from Human Protein Atlas, under the terms of the Creative Commons Attribution Share Alike License (CC BY SA). TEAD1, TEA domain transcription factor 1; TPM, transcripts per million; scRNA, single- cell RNA; mRNA, messenger RNA; tSNE, t-distributed stochastic neighbor embedding; CPM, counts per million.
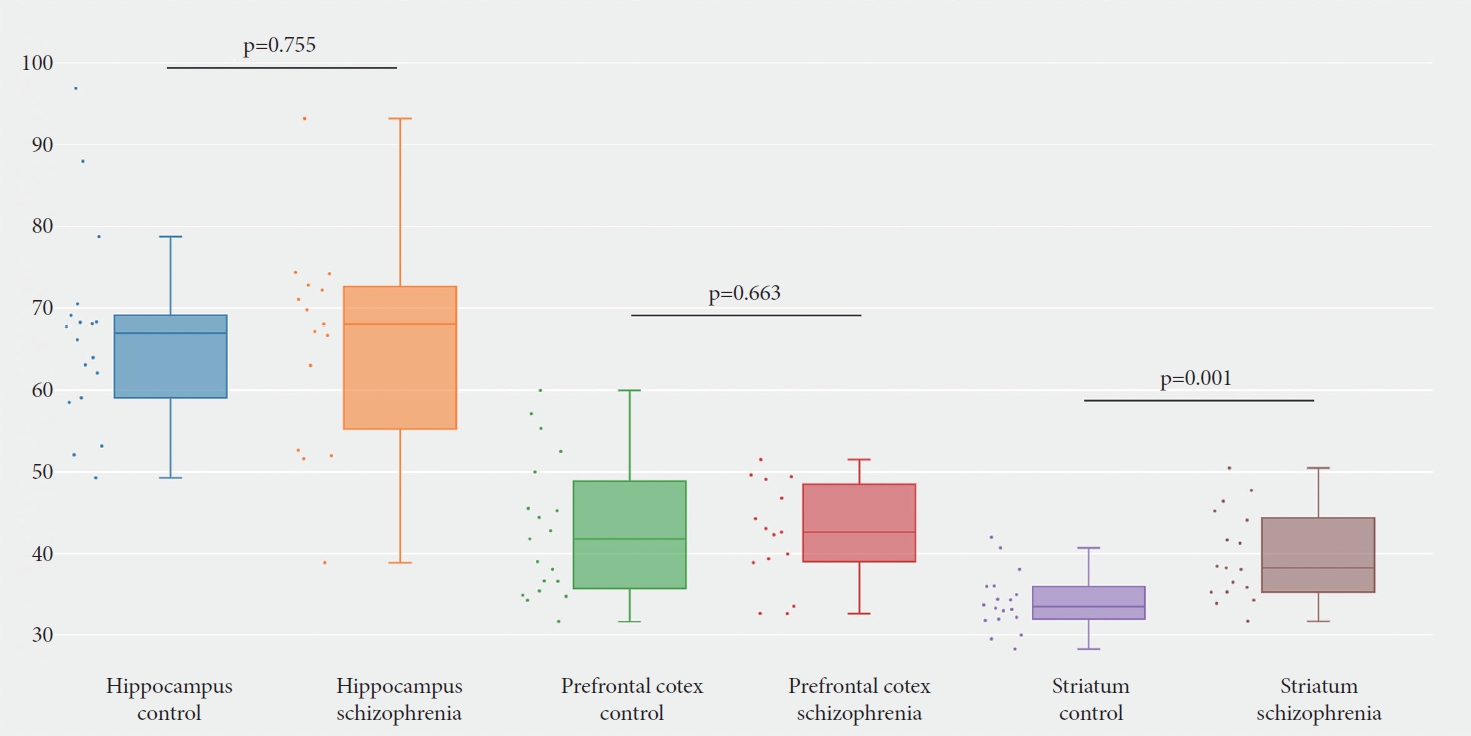
Expression difference of TEAD1 between cases and controls in three main schizophrenia-related brain regions. According to the SZDB database [23,24], a significant difference of the human TEAD1 gene expression was shown in stratum between the schizophrenia patients and healthy control subjects (corrected p=0.001).
We selected 9 SNPs in the TEAD1 gene region and performed allele and genotype association studies of these SNPs in a case–control sample consisted of 721 schizophrenia patients and 1,195 healthy controls. Detailed information and location of these selected SNPs are shown in Table 2, and none of the genotype distributions of these 9 SNPs in case and control groups deviated from Hardy-Weinberg equilibrium. Of the 9 SNPs, we found remarkable differences in both allele and genotype frequencies of four SNPs between cases and controls: rs6485989-SNP1 (allele: χ2=6.313, p=0.012; genotype: χ2=6.799, p=0.034), rs4415740-SNP2 (allele: χ2=11.564, p=0.001; genotype: χ2=13.024, p=0.002), rs7113256-SNP3 (allele: χ2=11.493, p=0.001; genotype: χ2=12.867, p=0.002), and rs1866709-SNP7 (allele: χ2=8.185, p=0.004; genotype: χ2=8.185, p=0.017) (Table 3). In addition, one SNP only showed significant association in allele frequency: rs12289262-SNP4 (χ2=4.838, p=0.028) (Table 3). After the Bonferroni correction, three SNPs remained significantly associated with schizophrenia in the allele or/and genotype frequencies: SNP2 (allelic p=0.006; genotypic p=0.014), SNP3 (allelic p=0.006; genotypic p=0.015) and SNP7 (allelic p=0.018) (Table 3).
Haplotype analysis of selected TEAD1 SNPs with schizophrenia
To further explore the association of haplotype structure, the standardized measure D’ value was used to evaluate pairwise linkage disequilibrium of the selected 9 SNPs of TEAD1. When D’ value of two SNPs were between 0.8 and 1.0, there is a strong linkage disequilibrium between them. We found three strong linkage disequilibrium haplotypes, which are shown in Figure 3.
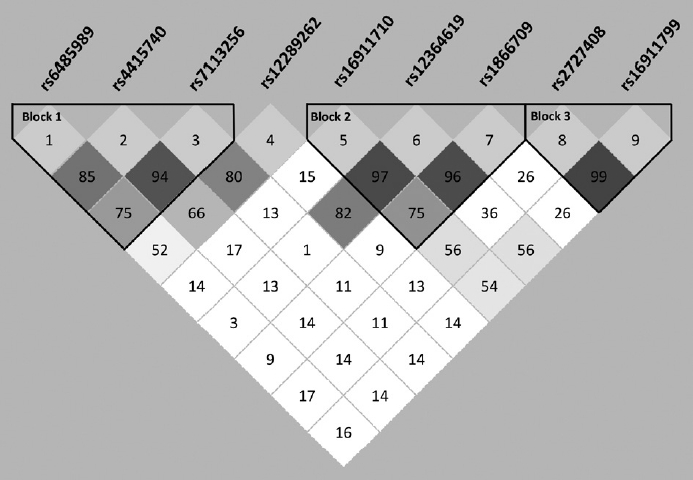
Linkage disequilibrium and haplotype block structure of the 9 selected TEAD1 SNPs. Linkage disequilibrium was computed for all possible combinations of the 9 SNPs using D’ values. Blocks were defined by a solid spine of linkage disequilibrium. Numbers in each box represent 100-fold of the D’ values between pairwise SNPs. TEAD1, TEA domain transcription factor 1; SNPs, single nucleotide polymorphisms.
To examine whether any haplotype contribute to a higher risk of schizophrenia, all haplotypes of the 9 SNPs were analyzed individually and globally. Specific p-values for individual haplotype combinations, global p-values for each haplotype and assessed haplotype frequencies in cases and controls are listed in Table 4. Among the three blocks, we found two blocks containing specific haplotype combinations related to schizophrenia. The first block includes three SNPs (rs6485989-SNP1, rs4415740-SNP2, and rs7113256-SNP3) which shows aggregated difference in frequency between cases and controls (global p=0.004). And individual haplotype combination also showed a significant difference between cases and controls (C-C-C: p=0.007; T-T-T: p<0.001). The second block which is constructed by three SNPs (rs16911710-SNP5, rs12364619-SNP6, and rs1866709-SNP7) also showed a distribution association with schizophrenia according to the global p-value or individual haplotypic p-values (global p=0.006; G-T-A: p=0.008; G-T-G: p=0.004).
DISCUSSION
In the present study, we investigated the association of human TEAD1 gene and its susceptibility to schizophrenia in a Northeast Chinese Han population. The 9 SNPs of human TEAD1 gene were selected and genotyped, and the results indicated that five SNPs (rs6485989, rs4415740, rs7113256, rs12289262, and rs1866709) were significantly associated with schizophrenia. Haplotype analysis showed significant difference in two strong LD blocks (rs6485989-rs4415740-rs7113256 and rs16911710-rs12364619-rs1866709) between case and control groups. Combining our results, it is suggested that the TEAD1 gene might be involved in susceptibility to schizophrenia.
Previous studies have demonstrated that TEAD1 is pivotal in neural development and neurological diseases. During neural development, TEAD1 is required for the proliferation of neural stem cells (NSCs) and the survival of NPCs [10,16]. Additionally, overexpression and knockdown experiments indicated that TEAD1 directly affects neural tube development, neuron generation, neuronal fate, cell apoptosis and cell migration [10,16,26]. TEAD1 also has demonstrable effects on neurological diseases. Mutation in TEAD1 will lead to Aicardi syndrome, a congenital neurodevelopmental disorder with intellectual disability, dilated cerebral ventricles, agenesis of the corpus callosum and neuronal migration disturbances [27]. Glioma is the most common primary brain tumor of the adult central nervous system. In the progression of glioma, long non-coding RNA miR-195-5p promotes cell proliferation, migration and invasion of glioma cells via YAP1-TEAD1-Hippo signaling [28]. Glioblastoma multiforme is a kind of glioma with the most malignant degree. The survival, progress and drug resistance of glioblastoma cells depend on their adaptation to low nutrition and hypoxia, in which low miR-124 levels contribute by directly regulating factors like TEAD1. That is, TEAD1 participates in cell survival and proliferation under stress in this tumor [29].
Recently, there is increasing evidence that dysconnectivity and functional imbalance of the striatum is involved in the pathophysiology of schizophrenia [30-33]. Dysregulation of dopaminergic function in striatum is the basis of many models that try to explain the underlying mechanism of schizophrenia symptoms [34]. Most schizophrenia patients are treated with antipsychotics, all of which basically depend on the blockade of dopamine D2 receptor in striatum. In addition, magnetic resonance imaging shows that the function and connection of striatum in schizophrenic patients are destroyed [35,36]. Individual differences in striatal circuits are one of the mechanisms of schizophrenia dysfunction, which affects treatment response [31]. In a study on biomarker, the striatum is the only brain region that can reliably predict the diagnostic status and antipsychotic response [37]. Moreover, evidence shows that polygenic risk of schizophrenia modulates striatal function [38,39]. The abnormal activation of striatum in schizophrenia is related to striatum dopaminergic system and the expression of risk genes for schizophrenia [37]. Our results revealed a difference of TEAD1 expression in stratum between the schizophrenia patients and healthy control subjects, indicating that TEAD1 may participate in the occurrence of schizophrenia.
The neurodevelopmental model of schizophrenia has become a hypothesis widely accepted in today’s research in schizophrenia. The two most sensitive stages for neurodevelopment are prenatal/perinatal period and adolescence, whose alterations may lead to the onset of schizophrenia. In the prenatal/perinatal period, there is no doubt that genes that participate in the proliferation, differentiation of NSCs and neuron migration will affect neural development. The activation of YAP-TEAD1 complex can lead to the proliferation of NSCs and NPCs because of their ability to promote cell cycle progression by up-regulation of cyclin D1 and to inhibit neuronal differentiation by down-regulation of NeuroM [16,26]. Mukhtar et al. [10] found that Tead1 overexpression significantly promoted differentiation of progenitors into Tbr1 + neurons and increased formation of deep cortical layer neurons in the cortical plate (CP). Furthermore, Tead1 overexpressing cells were significantly increased in the ventricular zone (VZ) and CP and reduced cells in the subventricular zone (SVZ)/intermediate zone (IZ) compared to controls, suggesting that TEAD1 caused the retention of cells in the VZ and premature migration from the SVZ/IZ to the CP. And then, dominant negative of Tead1 significantly increased retention of cells in the VZ and showed a reduced migration to the CP due to a defect in radial migration. Among the above-mentioned effects of TEAD1 on cell differentiation and neuron migration, apolipoproteins E, Disabled-2, and cysteine-rich protein 61 are direct targets of TEAD1 [10]. Nevertheless, in human glioblastoma, in vitro and in vivo experiments show that TEAD1 directly promote cell migration by regulating aquaporin 4 expression [17]. In addition to the regulation of neural development, TEAD1 is also important in the regulation of apoptosis, which possibly play a role in adolescence. The alteration of TEAD1 expression triggers apoptotic resistance by up-regulating the transcription of Livin, a member of the inhibitor of apoptosis protein family [40]. But the influence of TEAD1 on Livin expression is indirect, and one possible model is that TEAD1 activates an inhibitor of Livin by combining with a specific cofactor that gets titrated upon TEAD1 up-regulation [40]. Furthermore, through interaction with YAP, TEAD1 is able to positively activate the transcription of neuronal apoptosis inhibitory protein, a protein involved in apoptosis suppression [41]. On the whole, TEAD1 may affect susceptibility of schizophrenia by regulating pathways related to cell proliferation, differentiation, migration and apoptosis.
Our study first reported that TEAD1 is a susceptible gene of schizophrenia. There are several potential limitations of this result. First, our study was limited to Northeast China. More large-scale studies in more ethnic populations are required to verify the reliability of the association. Besides, the design of our study is a cross-sectional study so that the exact causal relationship between risk variants and schizophrenia cannot be concluded. In addition, biological functional analyses are required for further investigation on the role of TEAD1 in the pathogenesis of schizophrenia.
In conclusion, our case–control association study presented an association of the human TEAD1 gene with schizophrenia in a Northeast Chinese Han population, which may provide genetic evidence and promote further biological functional studies for the role of TEAD1 in the pathogenesis of schizophrenia and other mental diseases.
Notes
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Zhi-Lin Luan. Data curation: Yang Sun, Zhi-Lin Luan. Formal analysis: Yang Sun, Lin Wen. Funding acquisition: Zhi-Lin Luan. Methodology: Lin Wen, Yi-Yang Luo, Wen-Juan Hu, Cong Zhang, Ping Gao, Li-Na Xuan. Project administration: Zhi-Lin Luan. Resources: Yang Sun. Software: Ye Lv. Supervision: Zhi-Lin Luan. Validation: Guan-Yu Wang, Cheng-Jie Li, Zhi-Xin Xiang. Visualization: Yang Sun, Lin Wen. Writing—original draft: Yi-Yang Luo. Writing—review & editing: Zhi-Lin Luan, Hui-Wen Ren.
Funding Statement
This work was supported by Natural Science Foundation of Liaoning Province, China 2022-MS-326 (to Z.L.), Education Department of Liaoning Province, China LZ2020023 (to Z.L.), and the Dalian Young Star of Science and Technology 2019RQ116 (to Z.L.). We are also grateful for the support from Liaoning BaiQianWan Talents Program.
Acknowledgements
We extend our gratitude to all the patients participating in this study.