 |
 |
- Search
| Psychiatry Investig > Volume 20(10); 2023 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
Supplementary Materials
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Notes
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author Donghong Cui upon reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Ting Xue, Donghong Cui. Data curation: Hongna Huang, Yuan Shi, Xi Chen, Lijun Wang. Formal analysis: Hongna Huang. Funding acquisition: Donghong Cui. Investigation: Hongna Huang, Yuan Shi, Zifan Xiao, Xi Chen, Lijun Wang, Zezhi Li, Ting Xue. Methodology: Hongna Huang, Lizhao Du, Zhengping Pu, Shun Yao, Zezhi Li, Ting Xue, Donghong Cui. Project administration: Donghong Cui. Resources: Donghong Cui. Software: Hongna Huang. Supervision: Ting Xue, Donghong Cui. Validation: Donghong Cui. Visualization: Hongna Huang, Ting Xue, Donghong Cui. Writing—original draft: Hongna Huang, Lizhao Du, Zhengping Pu, Zifan Xiao. Writing—review & editing: Ting Xue, Donghong Cui.
Funding Statement
We would like to express our sincere gratitude to the patients and healthy controls who participated in this research. This work was supported by the National Key R&D Program of China (2017YFC0909200) and the National Natural Science Foundation of China (81671336, 82271544).
Figure 1.
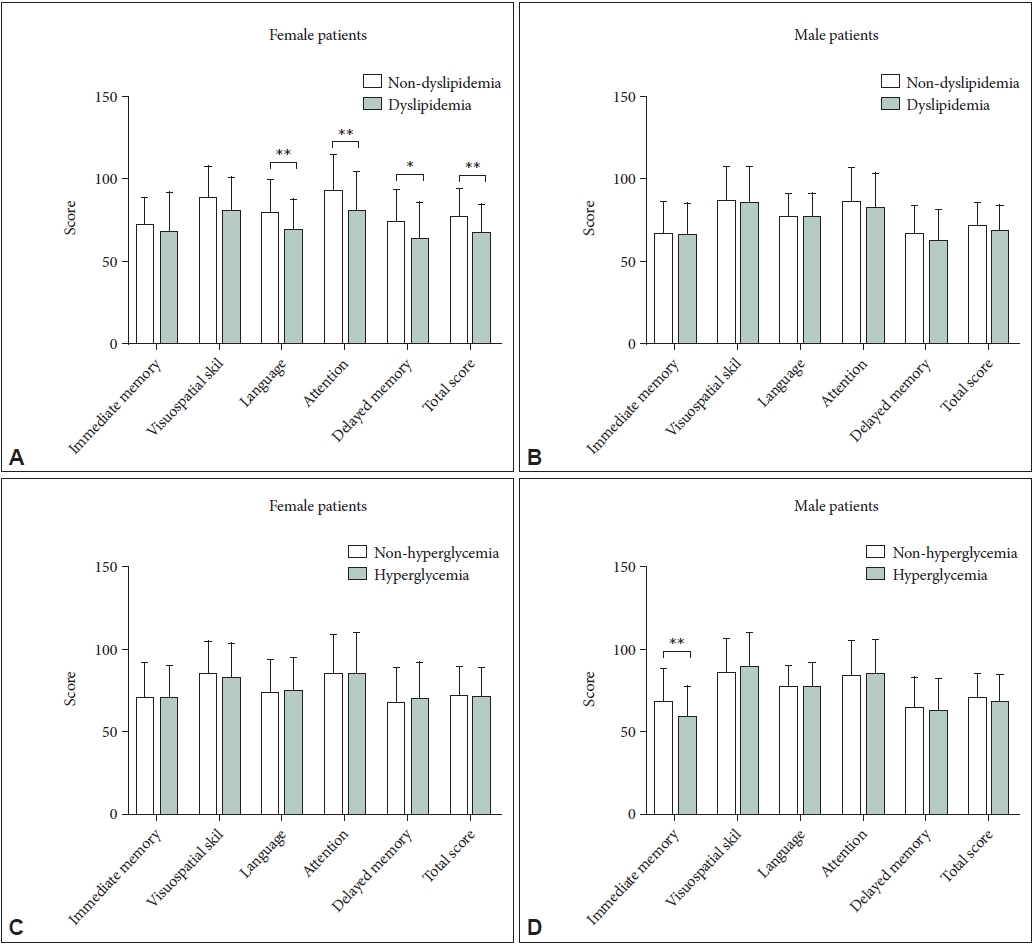
Table 1.
|
SZ (N=358) |
HC (N=231) |
Diagnosis |
Sex |
Diagnosis×sex |
||||
|---|---|---|---|---|---|---|---|---|
| Male (N=244) | Female (N=114) | Male (N=111) | Female (N=120) | F (p) | F (p) | F (p) | ||
| Age (yr) | 41.42±13.18 | 38.81±12.81 | 38.99±13.34 | 39.78±13.68 | 0.40 (0.528) | 0.63 (0.429) | 2.19 (0.139) | |
| Education (yr) | 10.70±3.49 | 10.79±4.30 | 14.64±4.15 | 14.76±4.65 | 121.88 (<0.001)*** | 0.09 (0.770) | 0.001 (0.973) | |
| BMI (kg/m2) | 24.42±3.67 | 23.87±4.42 | 23.23±2.37 | 21.59±2.26 | 34.53 (<0.001)*** | 13.91 (< 0.001)*** | 3.42 (0.065) | |
| RBANS | ||||||||
| Immediate memory | 65.20±21.21 | 68.41±21.01 | 81.75±16.93 | 81.34±19.54 | 19.69 (<0.001)*** | 0.87 (0.353) | 1.03 (0.311) | |
| Visuospatial skill | 86.43±21.83 | 83.72±21.15 | 101.25±14.33 | 99.21±12.93 | 26.96 (<0.001)*** | 1.45 (0.229) | 0.17 (0.684) | |
| Language | 75.97±15.52 | 72.58±21.62 | 92.39±14.71 | 89.50±17.61 | 41.44 (<0.001)*** | 4.70 (0.031)* | 0.06 (0.805) | |
| Attention | 82.84±22.97 | 84.52±26.31 | 110.86±14.51 | 112.34±16.77 | 100.03 (<0.001)*** | 1.17 (0.280) | <0.001 (0.982) | |
| Delayed memory | 63.96±19.72 | 67.94±22.03 | 88.60±16.28 | 91.86±15.16 | 108.80 (<0.001)*** | 7.27 (0.007)* | 0.004 (0.947) | |
| Total score | 69.07±16.48 | 70.75±18.76 | 92.40±13.42 | 92.98±16.13 | 116.02 (<0.001)*** | 1.22 (0.270) | 0.11 (0.737) | |
Table 2.
| Male (N=244) | Female (N=114) | t/χ2 | p | ||
|---|---|---|---|---|---|
| Age of onset (yr) | 24.66±7.91 | 26.40±9.45 | -1.74 | 0.082 | |
| Duration of illness (mo) | 208.19±146.23 | 152.90±135.07 | 3.26 | 0.001** | |
| Antipsychotic drugs | 5.27 | 0.071 | |||
| Typical | 14 (5.7) | 1 (0.9) | |||
| Atypical | 201 (82.4) | 103 (90.4) | |||
| Atypical+typical | 18 (7.4) | 7 (6.1) | |||
| PANSS score | |||||
| Positive symptom | 16.45±7.94 | 17.89±10.95 | -1.25 | 0.211 | |
| Negative symptom | 17.29±8.03 | 16.39±9.90 | 0.84 | 0.401 | |
| General psychopathology | 32.83±12.77 | 35.33±18.43 | -1.31 | 0.193 | |
| Total score | 66.55±25.24 | 69.61±36.17 | -0.82 | 0.416 | |
| Metabolic indexes | |||||
| Waist circumference (cm) | 90.83±10.25 | 85.75±12.51 | 3.49 | 0.001** | |
| Systolic BP (mm Hg) | 121.41±12.40 | 115.72±10.46 | 4.23 | <0.001*** | |
| Diastolic BP (mm Hg) | 77.93±8.66 | 74.28±6.90 | 3.94 | <0.001*** | |
| Fasting glucose (mmol/L) | 5.25±1.16 | 5.22±1.72 | 0.17 | 0.863 | |
| Triglycerides (mmol/L) | 1.63±0.94 | 1.56±0.93 | 0.67 | 0.502 | |
| HDLC (mmol/L) | 1.10±0.28 | 1.34±0.34 | -6.36 | <0.001*** | |
Table 3.
| Male (N=244) | Female (N=114) | B | Wald χ2 | OR | 95% CI | p | |
|---|---|---|---|---|---|---|---|
| Abdominal obesity | 131 (58.5) | 63 (66.3) | -1.05 | 8.36 | 0.35 | 0.17-0.71 | 0.004** |
| Hypertension | 99 (40.6) | 24 (21.2) | 0.93 | 10.20 | 2.53 | 1.43-4.48 | 0.001** |
| Hyperglycemia | 68 (28.8) | 25 (22.7) | 0.04 | 0.02 | 1.04 | 0.58-1.86 | 0.894 |
| Dyslipidemia | 147 (61.0) | 67 (59.8) | -0.12 | 0.22 | 0.89 | 0.53-1.47 | 0.638 |
| MetS | 83 (38.1) | 29 (31.9) | 0.07 | 0.05 | 1.07 | 0.59-1.96 | 0.818 |
Table 4.
| MetS | Abdominal obesity | Hypertension | Hyperglycemia | Dyslipidemia | |
|---|---|---|---|---|---|
| Immediate memory | |||||
| Male | -0.106 | -0.054 | 0.009 | -0.204* | -0.008 |
| Female | -0.128 | -0.079 | -0.124 | 0.010 | 0.034 |
| Visuospatial skill | |||||
| Male | 0.007 | -0.014 | 0.005 | 0.059 | -0.030 |
| Female | -0.006 | -0.078 | 0.084 | -0.074 | -0.092 |
| Language | |||||
| Male | -0.022 | 0.050 | 0.020 | -0.025 | -0.026 |
| Female | -0.003 | 0.106 | 0.035 | -0.004 | -0.199* |
| Attention | |||||
| Male | -0.022 | -0.156 | 0.018 | 0.021 | -0.062 |
| Female | -0.065 | 0.001 | 0.100 | -0.018 | -0.191* |
| Delayed memory | |||||
| Male | -0.028 | -0.001 | -0.008 | -0.080 | -0.080 |
| Female | -0.045 | -0.065 | 0.117 | -0.014 | -0.243* |
| Total score | |||||
| Male | -0.031 | -0.049 | 0.019 | -0.060 | -0.054 |
| Female | -0.063 | -0.023 | 0.057 | -0.028 | -0.195* |
REFERENCES







